Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT) Following Adenovirus Vector COVID-19 Vaccination: Interim Guidance for Healthcare Professionals in the Outpatient Setting
Authors:Menaka Pai, Allan Grill, Noah Ivers, Nathan M. Stall, Katherine J. Miller, Ullanda Niel, Benjamin Chan, Antonina Maltsev, Ayodele Odutayo, Fahad Razak, Michael Schull, Brian Schwartz, Michelle Sholzberg, Robert Steiner, Sarah Wilson, Peter Jüni, Andrew M. Morris on behalf of the Drugs & Biologics Clinical Practice Guidelines Working Group and the Ontario COVID-19 Science Advisory Table
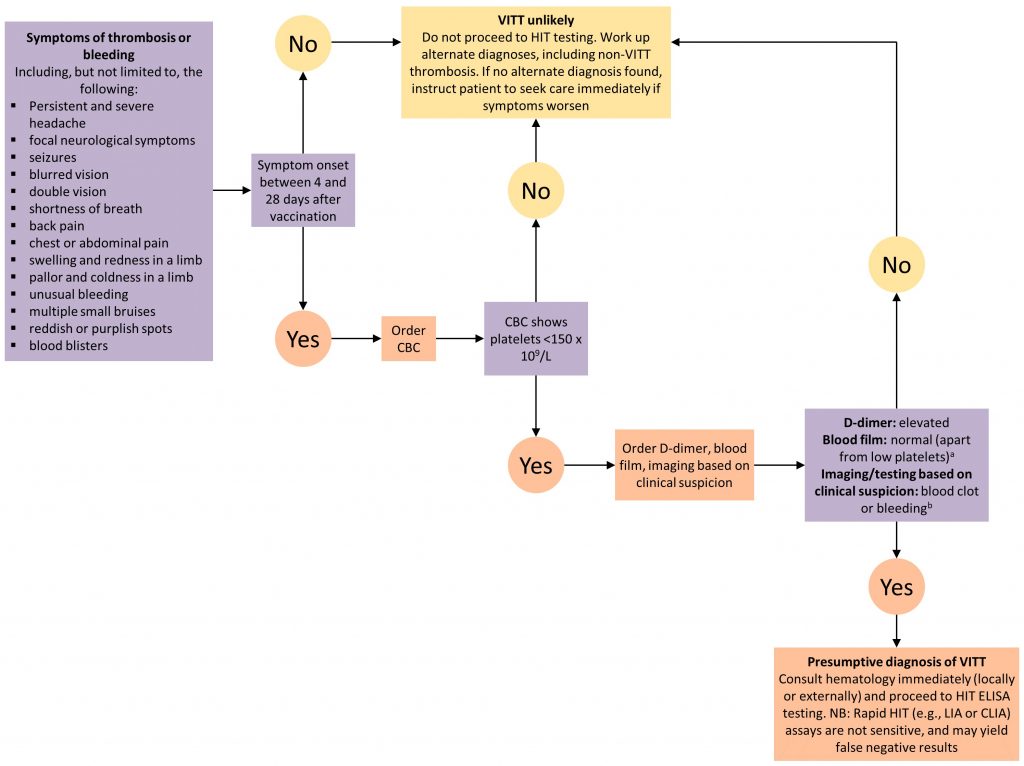
Figure 1. Decision Tree for Diagnosing and Ruling Out VITT
aBlood film to rule out platelet clumping as a cause of low platelet count; bNot all cases of VITT initially present with a clot or bleeding. Patients with all of the features of presumptive VITT (low platelets, high D-dimer, presenting 4 to 28 days post-vaccination) but NO blood clot or bleeding merit hematology consultation (locally or externally) to consider starting treatment until the results of confirmatory testing are available. VITT, vaccine-induced immune thrombotic thrombocytopenia. CBC, complete blood count. HIT, heparin induced thrombocytopenia. ELISA, enzyme linked immunosorbent assay. LIA, latex immunoturbidometric assay. CLIA, chemiluminescent immunoassay.
What do we know so far?
Adenoviral vector COVID-19 vaccines, including the AstraZeneca/COVISHIELD vaccine and the Janssen/Johnson & Johnson vaccine, are associated with immune thrombosis that is similar to heparin-induced thrombocytopenia (HIT). Women and young people appear to be slightly overrepresented in reported cases, and thrombosis seems to occur 4 to 28 days after vaccination. Affected individuals have antibodies targeted against platelet factor 4 (PF4) that induce massive platelet activation, reducing the platelet count and causing thrombosis.1–3 This phenomenon is similar to HIT, but, unlike HIT, VITT does not require heparin as a trigger. It has been referred to as Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT), Vaccine-Induced Prothrombotic Immune Thrombocytopenia (VIPIT), and Thrombosis Thrombocytopenia syndrome (TTS). In this Science Brief, the term VITT will be used. Published estimates of the incidence of VITT range from 1 case per 26,000 to 1 case per 127,000 doses of AstraZeneca/COVISHIELD administered.3–6 There have been few reported cases of VITT with the Janssen/Johnson & Johnson vaccine thus far, so it is challenging to calculate a precise frequency, but the incidence of VITT appears to be approximately 1 case per 500,000 vaccine doses administered.7
Are certain patients predisposed to VITT?
At this time, it is not clear if certain patients are predisposed to VITT. Early reported cases were predominantly in younger women, however these individuals may have been overrepresented in the vaccinated population in reporting countries. Cases have now been reported in men and in older adults. Since VITT appears immune-mediated and linked to a very specific antigen, an individual with classical risk factors for blood clots, including thrombophilia, a family history of blood clots, a personal history of arterial or venous clots, autoimmune disease, low platelets without a history of clotting, a disorder of platelet function, or who is on birth control or other hormones, or who is pregnant, is probably not at increased risk of VITT. Accordingly, there are no new contraindications to receiving the AstraZeneca/COVISHIELD or Janssen/Johnson & Johnson COVID-19 vaccines. However, Health Canada recommends that individuals who have experienced a previous cerebral sinus vein thrombosis (CVST) with thrombocytopenia or HIT should only receive these vaccines if the potential benefits outweigh the potential risks; they may be at increased risk of VITT.8,9
The National Advisory Committee on Immunization (NACI) makes a strong preferential recommendation for mRNA vaccines for all Canadians. NACI has recommended that the AstraZeneca/COVISHIELD or Janssen/Johnson & Johnson vaccines may be offered to Canadians 30 years of age and older, if the benefits outweigh risks of waiting for an mRNA vaccine, the decision to receive the vaccine is informed by risks and consequences of VITT, and the delay to receive an mRNA vaccine is substantial.10 NACI outlines that risk-benefit decisions should be informed by several factors including the local COVID-19 epidemic conditions, local vaccine supply, an individual’s risk of severe illness and death if they develop COVID-19, and their risk of exposure to the SARS-CoV-2 virus.
What should primary care providers and patients look out for post-vaccination?
Patients with VITT may present with CSVT, or with other arterial or venous clots or bleeding. Some symptoms make it more likely that a patient has VITT: persistent and severe headache, seizures, or focal neurological symptoms including blurred or double vision (suggesting CSVT or arterial stroke); shortness of breath, chest, back, or abdominal pain (suggesting pulmonary embolism, acute coronary syndrome, abdominal vein thrombosis, or adrenal hemorrhage); unusual bleeding, bruising, petechiae, or blood blisters (suggesting thrombocytopenia or disseminated intravascular coagulation); or limb swelling, redness, pallor, or coldness (suggesting deep vein thrombosis or acute limb ischemia). VITT seems to occur between 4 to 28 days post-vaccination. Symptoms that begin in this time frame should raise the clinical suspicion of VITT.
What should primary care providers and patients do if concerning symptoms arise?
All patients with unusual, non-severe symptoms following vaccination should have an assessment (virtual or in-person) with their primary care provider, and a diagnosis of VITT should be considered; initial investigations may be done in the primary care setting. Patients with severe symptoms should immediately present to the nearest emergency department.
Clinicians should ask patients about their COVID-19 vaccine history and should draw a complete blood count (CBC). VITT is unlikely if symptom onset falls outside of the 4 to 28 day time frame OR if the platelet count is ≥ 150 x 109/L.1–3,11,12 VITT is more likely if symptom onset falls within the 4 to 28 day time frame AND the platelet count is < 150 x 109/L, and such patients should be evaluated at their nearest emergency department for suspected VITT. This will expedite further diagnostic workup, treatment, and urgent hematology consultation (local or external).
Treatment principles for patients with presumptive and confirmed VITT are summarized below.

Summary Box. Treating Patients with Presumptive or Confirmed VITT
*Platelet transfusions could theoretically worsen the clotting; if patients present with a life-threatening bleed, platelets should only be transfused under the guidance of a hematologist.
Is VITT a reportable event?
All suspected adverse events following immunization (AEFI), including thrombosis, thrombocytopenia, and both presumptive and confirmed VITT, should be reported using the provincial AEFI form and sent to the local Public Health Unit. More information on how to report AEFIs can be found on the Public Health Ontario website. Ontario conducts vaccine surveillance safety in collaboration with the Public Health Agency of Canada, and prompt reporting is essential to learn more about this rare but serious thrombotic phenomenon.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. Published online April 9, 2021. https://doi.org/10.1056/NEJMoa2104840
- Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. Published online April 16, 2021. https://doi.org/10.1056/NEJMoa2105385
- Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. Published online April 9, 2021. https://doi.org/10.1056/NEJMoa2104882
- Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. https://doi.org/10.1136/bmj.n1114
- Government of United Kingdom. Coronavirus vaccine – weekly summary of Yellow Card reporting. GOV.UK. Published April 29, 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
- Australian Government. COVID-19 vaccine weekly safety report – 06-05-2021. Therapeutic Goods Administration (TGA). Published May 6, 2021. Accessed May 7, 2021. https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-06-05-2021
- Oliver S. Risk/Benefit Assessment of Thrombotic Thrombocytopenic Events after Janssen COVID-19 Vaccines. Advisory Committee on Immunization Practices (ACIP); 2021:76. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-04-23/06-COVID-Oliver-508.pdf
- Health Canada. Product Monograph Including Patient Medication Information: AstraZeneca COVID-19 Vaccine. Government of Canada; 2021:26. https://covid-vaccine.canada.ca/info/pdf/astrazeneca-covid-19-vaccine-pm-en.pdf
- Health Canada. Product Monograph Including Patient Medication Information: Janssen COVID-19 Vaccine. Government of Canada; 2021:24. https://covid-vaccine.canada.ca/info/pdf/janssen-covid-19-vaccine-pm-en.pdf
- Advisory Committee Statement (ACS). Recommendations on the Use of COVID-19 Vaccines. National Advisory Committee on Immunization (NACI); 2021:120. https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/recommendations-use-covid-19-vaccines-en.pdf
- Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res Pract Thromb Haemost. Published online September 25, 2020. https://doi.org/10.1002/rth2.12439
- Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of vaccine-induced immune thrombotic thrombocytopenia (VITT) for SARS-CoV-2 infections: Communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. Published online April 22, 2021. https://doi.org/10.1111/jth.15341
Citation: Pai M, Grill A, Ivers N, et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT) following adenovirus vector COVID-19 vaccination: interim guidance for healthcare professionals in the outpatient setting. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2021;2(20). https://doi.org/10.47326/ocsat.2021.02.20.2.0
Author Affiliations: The affiliations of the members of the Ontario COVID-19 Science Advisory Table can be found at https://covid19-sciencetable.ca/.
Declarations of Interest: The declarations of interest of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners can be found at https://covid19-sciencetable.ca/. The declarations of interest of external authors can be found under Additional Resources.
Copyright: 2021 Ontario COVID-19 Science Advisory Table. This is an open access document distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original work is properly cited.
The views and findings expressed in this Science Brief are those of the authors and do not necessarily reflect the views of all of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners.
