Baricitinib for Hospitalized Patients with COVID-19
Authors:Amanda K. Hempel, Stefan D. Jevtic, Stacey Vandersluis, Pavlos Bobos, Stephanie Carlin, William Ciccotelli, Elizabeth Leung, Peter Jüni, Noel Rosen, Liliana Volnikova, Anupma Wadhwa, Andrew M. Morris, Menaka Pai on behalf of the Drugs & Biologics Clinical Practice Guidelines Working Group and the Ontario COVID-19 Science Advisory Table.
Key Message
Baricitinib, a Janus-associated kinase (JAK) inhibitor, reduces mortality and may reduce progression to mechanical ventilation in COVID-19 patients, with no increase in serious adverse events.
Baricitinib should be used in moderately ill patients (i.e., requiring supplemental oxygen via nasal prongs) and critically ill hospitalized patients (i.e., requiring oxygen via high-flow nasal cannula, non-invasive ventilation (NIV), invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation (ECMO)) with COVID-19 who are on recommended doses of dexamethasone (or another dose-equivalent corticosteroid) or who have a contraindication to corticosteroids. Combined use of baricitinib and interleukin-6 (IL-6) inhibitors is not recommended due to a lack of efficacy and safety data. Decisions regarding the use of baricitinib versus an IL-6 inhibitor should be made based on clinical judgement and patient preference regarding availability, side effects and contraindications.
The baricitinib dose should be 4 mg PO or NG (2 x 2 mg tablets) daily for 14 days or until discharge. This should be reduced to 2 mg daily in patients with an eGFR 30-60 mL/min/1.73m2. It should not be used in patients with eGFR <30 mL/min/1.73m2.
Lay Summary
How Baricitinib Works
Baricitinib is a small molecule that is already approved by Health Canada for treating rheumatoid arthritis (RA), available in tablet form. Baricitinib works by blocking Janus kinase, one of our body’s signaling proteins, which is involved in the inflammatory response to infections such as COVID-19. It may also directly inhibit the virus that causes COVID-19 from entering into our body’s cells.
Baricitinib Can Save Lives in COVID-19 Patients with Moderate or Critical Illness
Patients with the most severe cases of COVID-19 develop dangerous inflammation that can worsen their breathing and damage their lungs, heart, kidneys, and other vital organs.
As a COVID-19 treatment, baricitinib should be used in patients with COVID-19 whom doctors determine to be “moderately” to “critically” ill. Patients receiving baricitinib should already be receiving corticosteroids (a class of drugs that also reduce inflammation) and supplemental oxygen. Moderately or critically ill patients may have already received an IL-6 inhibitor (a class of drugs that further reduces the inflammatory response); there have been no studies examining baricitinib in combination with IL-6 inhibitors. Based on their mechanisms of action, combining these agents may result in greater risk of side effects. At this time combined use of baricitinib and IL-6 inhibitors is not recommended. There have been no studies comparing baricitinib and IL-6 inhibitors. Decisions regarding whether to treat with baricitinib or an IL-6 inhibitor should be made by the doctor and patient taking into consideration availability, side effects and contraindications. Baricitinib should not be used in patients who have other bacterial, fungal or viral infections, including tuberculosis, or who have dramatically reduced kidney function.
How We Came to Our Recommendations
To understand baricitinib’s effect on moderately or critically ill patients with COVID-19, we reviewed the available published evidence. Three randomized controlled trials (RCTs) compared baricitinib to placebo in hospitalized patients who were also receiving routine care. When their results were taken together, the studies found that baricitinib can decrease death and decrease the chance that a hospitalized COVID-19 patient will need invasive mechanical ventilation. There is no benefit in mild COVID-19 patients who were hospitalized and no evidence was available in mildly ill patients who were not hospitalized.
Patients with COVID-19 receiving baricitinib in these studies very rarely had side effects, and no more likely to experience serious side effects than patients in the placebo groups.
Our Recommendations for Using Baricitinib as a Treatment for COVID-19
We recommend that patients with COVID-19 receive baricitinib if they have moderate or critical illness requiring supplemental oxygen – whether it is low-flow oxygen through nasal prongs, higher-flow oxygen through external devices, or IMV with a breathing tube. Before patients receive baricitinib, they should also be receiving recommended doses of dexamethasone or an equivalent corticosteroid, a drug that reduces inflammation, unless they are unable to take corticosteroids for any reason.
The panel does not recommend combined use of baricitinib and IL-6 inhibitors (a different class of drugs that reduce inflammation) for COVID-19 due to absence of safety and efficacy evidence.
At this time, we do not recommend baricitinib for patients who have mild illness.
Summary
Background
Some patients with progressive COVID-19 experience an excessive immune response that results in severe organ injury. Anti-inflammatory agents, such as dexamethasone and IL-6 inhibitors (e.g., tocilizumab and sarilumab), have demonstrated a mortality benefit in patients with COVID-19, supporting the concept that other anti-inflammatory agents may be effective as well. Baricitinib is currently approved by Health Canada for treatment of RA. It is a small molecule inhibitor of JAK, a protein involved in the production of inflammatory cytokines. Additionally, it is hypothesized that baricitinib may prevent viral entry into cells.
Questions
Does baricitinib improve patient outcomes such as mortality, need for mechanical ventilation or organ support, resolution of symptoms, and adverse events for individuals hospitalized with COVID-19?
Which hospitalized COVID-19 patients will receive the most benefit from baricitinib?
Findings
As of November 1, 2021, three RCTs have been published, either as preprints or in peer-reviewed journals: ACTT-2, COV-BARRIER and COV-BARRIER addendum trials. These trials studied the effects of baricitinib compared to placebo alongside standard of care, in a total of 2,659 patients hospitalized with COVID-19. Patients enrolled in these trials had illness severity ranging from moderately to critically ill.
Meta-analysis of the RCTs demonstrates that baricitinib reduces mortality compared to placebo (risk ratio (RR) 0.64, 95% confidence interval (CI) 0.51 – 0.80). Findings were driven by a statistically significant difference amongst critically ill patients receiving high-flow oxygen (HFO) or NIV (RR 0.59, 95% CI 0.42 – 0.85) as well as moderately ill patients requiring supplemental oxygen (RR 0.62, 95% CI 0.41 – 0.94). There was also a signal towards benefit for critically ill patients requiring IMV or ECMO (RR 0.77, 95% CI 0.51 – 1.15), however this was not statistically significant. There is uncertainty about the potential benefit for mildly ill hospitalized patients (not requiring oxygen).
Findings were inconsistent regarding whether the use of baricitinib reduced the need for mechanical ventilation or organ support. In the ACTT-2 trial, baricitinib reduced the progression to IMV or ECMO (RR 0.64, 95% CI 0.44 – 0.93), and the composite outcome of IMV or mortality (RR 0.69, 95% CI 0.50 – 0.95). However, findings in ACTT-2 were not significant for progression to NIV or HFO, nor in COV-BARRIER for the composite outcome of progression to HFO, NIV, IMV or death.
Baricitinib was found to decrease the time to recovery in patients requiring HFO or NIV from 18 to 10 days in the ACTT-2 trial but was not significant in any other subgroup. Baricitinib did not show a statistically significant difference in rate of recovery but trended in favour of those receiving baricitinib (RR 1.03, 95% CI 0.99 – 1.07). As well, there was a reduced rate of serious adverse events among patients who received baricitinib as compared to placebo in all three RCTs (RR 0.78, 95% CI 0.67 – 0.91).
Practical Considerations
Patients who are pregnant or breastfeeding were not included in the above trials. Use in these populations should be determined through shared decision making with the patient to evaluate potential maternal benefits and theoretical fetal risks.
Additionally, the baricitinib dose should be reduced to 2 mg daily in patients with an eGFR 30-60 mL/min/1.73m2. It should not be used in patients with eGFR <30 mL/min/1.73m2.
Patients receiving baricitinib should have a complete blood count, creatinine function and liver enzymes monitored throughout the treatment duration.
Recommendations
Please see the section below entitled “Methods Used for this Scientific Brief” for a description of COVID-19 illness severity criteria.
Critically Ill Patients
Baricitinib 4 mg PO or NG daily (2 x 2 mg tablets) for 14 days or until discharge may be considered in patients receiving non-invasive or high-flow nasal oxygen, mechanical ventilation or ECMO who are on a recommended dose of dexamethasone therapy (or a dose-equivalent corticosteroid) or who have a contraindication to corticosteroid treatment.
The panel does not recommend combined use of baricitinib and IL-6 inhibitors due to absence of safety and efficacy evidence.
Moderately Ill Patients
Baricitinib 4 mg PO daily (2 x 2 mg tablets) for 14 days or until discharge may be considered in patients on supplemental oxygen who are on a recommended dose of dexamethasone therapy (or a dose-equivalent corticosteroid) or who have a contraindication to corticosteroid treatment.
The panel does not recommend combined use of baricitinib and IL-6 inhibitors due to absence of safety and efficacy evidence.
Mildly Ill Patients
Baricitinib is not recommended in mildly ill COVID-19 patients who do not require oxygen.
Background
COVID-19 is a viral infection that can result in severe, systemic inflammation in certain patients. This inflammation, sometimes referred to as “cytokine storm”, is responsible for many of the clinical sequelae associated with COVID-19.1 These complications include acute respiratory distress syndrome (ARDS), thrombosis, and multi-organ failure. Anti-inflammatory agents, such as dexamethasone and IL-6 inhibitors have demonstrated significant improvement in mortality for COVID-19 patients, likely by limiting excess inflammation. Artificial intelligence algorithms have suggested that similar anti-inflammatory molecules, such as baricitinib, may offer additional benefit.2
Baricitinib is a small molecule JAK inhibitor that is Health Canada-approved for use in treatment of moderate to severe RA. It reduces inflammation via inhibition of the JAK-STAT pathway in cells. Normally, JAK proteins are involved in transduction of cell surface receptor signaling through activation of STAT proteins. Once activated, STAT proteins mediate nuclear signaling to promote inflammatory gene transcription, including molecules such as interferon-1 and IL-6. Baricitinib reversibly inhibits JAK1 and JAK2, thus preventing this inflammatory cytokine production.
In addition to this anti-inflammatory activity, baricitinib is hypothesized to exert direct antiviral effects by preventing viral entry into cells.2–4 SARS-CoV-2 enters cells by binding to the ACE2 receptor on cell surfaces and activating several intracellular proteins. AP2-associated protein kinase 1 (AAK1) is one such protein that is inhibited by baricitinib in vitro.5 Based on these mechanisms of action, baricitinib is hypothesized to improve outcomes in COVID-19 patients in addition to current standard therapy. Unlike IL-6 inhibitors, which also act directly on the cytokine pathway, baricitinib has a relatively short half-life (elimination half-life of ~12 hours) which has theoretical benefit in minimizing the duration of immunosuppression. At this time, there is insufficient data to demonstrate whether this translates to a reduction of opportunistic infections.
Questions
Does baricitinib improve patient outcomes such as mortality, need for mechanical ventilator or organ support, resolution of symptoms and adverse events for individuals hospitalized with COVID-19?
Which hospitalized COVID-19 patients will receive the most benefit from baricitinib?
Findings
At the time of writing, we are aware of three RCTs published that evaluated baricitinib compared to placebo alongside standard of care. The studies were large international efforts with recruitment between July 2020 and April 2021 including a collective total of 2,659 patients. The trials had similar patient populations and used the same scale to stratify severity. The primary difference between populations was the low use of concomitant corticosteroids in the ACTT-2 trial as compared to the COV-BARRIER trial and its addendum. The studies included mild, moderate, and critically ill patients (see Methods section for definitions).
ACTT-2
This randomized, multi-centre trial compared the use of baricitinib plus remdesivir to placebo plus remdesivir in hospitalized patients with polymerase chain reaction (PCR)-confirmed COVID-19 and evidence of lower-respiratory tract infection. Findings were published online December 11, 2020.6
The trial enrolled 1,033 patients between May 8, 2020 and July 1, 2020 in 8 countries. This included 142 patients without oxygen, 564 patients on supplemental oxygen, 216 patients on NIV or HFO, and 11 patients on IMV or ECMO. Participants could only receive additional treatments that were part of the participating hospital’s standard of care, and 11.9% received corticosteroids.
The primary outcome was time to recovery, defined as the first day on which patients were either discharged or no longer requiring medical care up to day 28. Secondary outcomes included clinical status, clinical improvement, mortality, time to discharge, new oxygen use, progression to IMV or ECMO, progression to NIV or HFO, duration of hospitalization and adverse events.
COV-BARRIER
This randomized, multi-centre trial compared the use of baricitinib vs. placebo in hospitalized patients receiving standard of care. Eligible patients had at least one elevated inflammatory marker and were not requiring IMV at enrolment. Following disclosure of results from the ACTT-2 trial, enrolment was limited to participants requiring baseline oxygen support starting October 20, 2020. Findings were published online September 1, 2021.7
The trial enrolled 1,525 patients between June 11, 2020 and January 15, 2021 in 12 countries. This included 186 patients not requiring oxygen, 962 patients requiring supplemental oxygen, and 370 patients on NIV or HFO. At baseline 79% were receiving systemic corticosteroids and 19% were receiving remdesivir as part of local standard of care. Patients using tocilizumab, convalescent plasma or neutralizing antibodies were excluded.
The trial used a composite primary endpoint of progression to HFO or NIV, IMV or ECMO, or death by day 28. A secondary endpoint was 28-day all-cause mortality. The trial also considered other key secondary outcomes as 60-day all-cause mortality, clinical improvement, ventilator-free days, time to recovery, and improvement in oxygen saturation.
COV-BARRIER Addendum
This addendum trial was a randomized, multicenter trial comparing the use of baricitinib to placebo in addition to standard of care in COVID-19 patients requiring IMV or ECMO, a population that was not included in the original COV-BARRIER study.7 Findings were released as a preprint online on October 12, 2021.8
The trial enrolled 101 participants between December 23, 2020 and April 10, 2021 in 4 countries. As in the main trial, patients were required to have at least one elevated inflammatory marker. At baseline 86% received systemic corticosteroids and 2% received remdesivir. Patients receiving tocilizumab, convalescent plasma or neutralizing antibodies were excluded.
Outcomes included 28-day and 60-day all-cause mortality, ventilator-free days, time to and rate of clinical improvement, duration of hospitalization and time to recovery.
Outcomes
All-Cause Mortality
Baricitinib reduced overall 28-day mortality compared to placebo (RR 0.64, 95% CI 0.51 – 0.80). This translates to an absolute risk reduction of 6%, meaning one additional death was prevented per 18 baricitinib-treated patients (Figure 1).
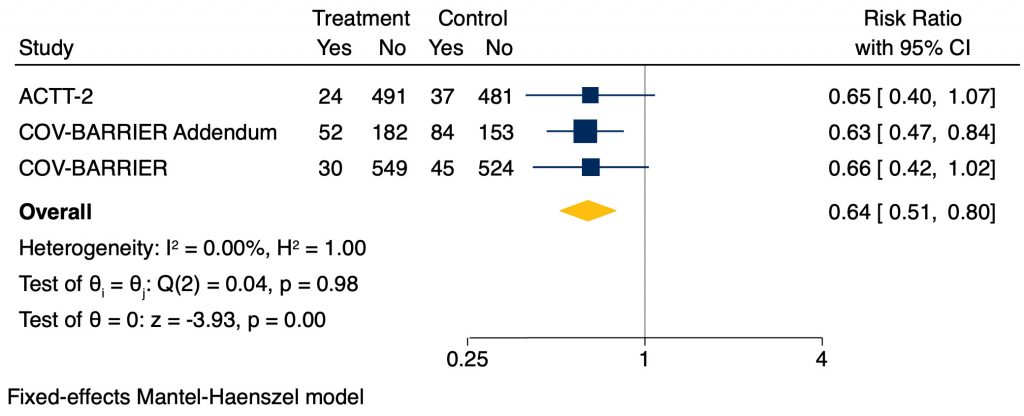
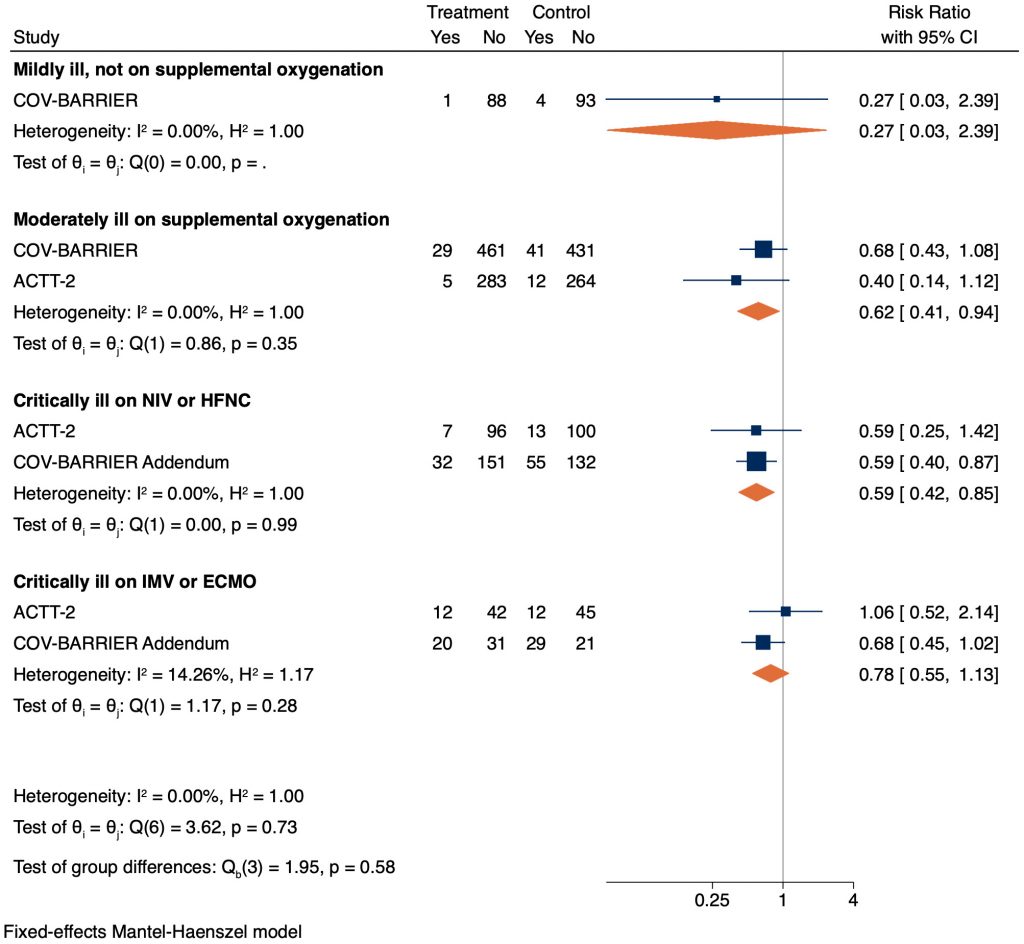
Baricitinib reduced mortality amongst critically ill patients receiving NIV or HFO (RR 0.59, 95% CI 0.42 – 0.85). This translates to an absolute risk reduction of 8%, meaning one additional death was prevented per 13 baricitinib-treated patients. In critically ill patients on IMV or ECMO there was a signal towards reduction in mortality, but this was not statistically significant (RR 0.78, 95% CI 0.55 – 1.13; Figure 2).
Baricitinib reduced mortality amongst moderately ill patients on supplemental oxygen, as compared to placebo, with a RR of 0.62 (95% CI 0.41 – 0.94). This corresponds to an absolute risk difference of 3%, meaning one additional death was prevented per 33 baricitinib-treated patients. However, there was uncertainty for mildly ill patients (not requiring supplemental oxygen) due to a very wide confidence interval and lack of statistical significance (RR 0.27, 95% CI 0.03 – 2.39; Figure 2).
Invasive Mechanical Ventilation or Organ Support
In the ACTT-2 trial, baricitinib reduced the risk of progression to new invasive ventilation or ECMO with an overall RR of 0.64 (95% CI 0.44 – 0.93) in the overall population.6 Baricitinib did not significantly reduce the use of new NIV or high flow oxygen, with an overall RR of 0.82 (95% CI 0.60 – 1.13). The COV-BARRIER trial did not report progression to IMV or ECMO.7,8
Invasive Mechanical Ventilation or Mortality (Composite Outcome)
Patients who die may not proceed to IMV before death. Therefore, it is important to consider the composite outcome of mortality or progression to IMV. In the ACTT-2 trial, baricitinib reduced the progression to death or IMV with a RR of 0.69 (95% CI 0.50 – 0.95).6 In the COV-BARRIER trial, there was a trend towards reduction of the primary outcome of progression to HFO, NIV, IMV or death with an odds ratio (OR) of 0.85 (95% CI 0.67 – 1.08).7,8 However, this was not statistically significant. We chose not to meta-analyze this outcome because the two trials used different criteria for their composite outcomes.
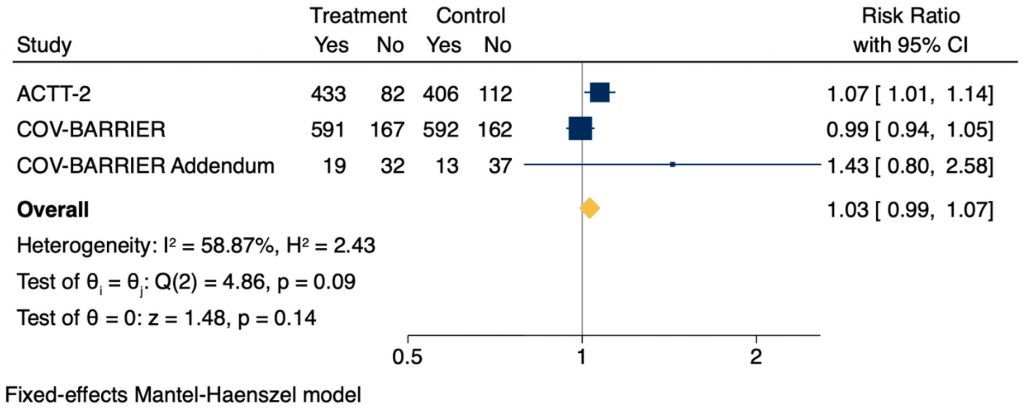
Recovery
Baricitinib did not show a statistically significant difference in recovery within 28 days, defined as no longer hospitalized or no longer requiring medical care, but trended in favour of those receiving baricitinib (RR 1.03, 95% CI 0.99 – 1.07; Figure 3). In the ACTT-2 trial, baricitinib also significantly improved time to recovery in patients requiring HFO or NIV from 18 days to 10 days (RR 1.51, 95% CI 1.10 – 2.08), however there was no significant improvement noted in any other severity subgroup.6 There was no significant difference in duration of hospitalization in any of the trials.
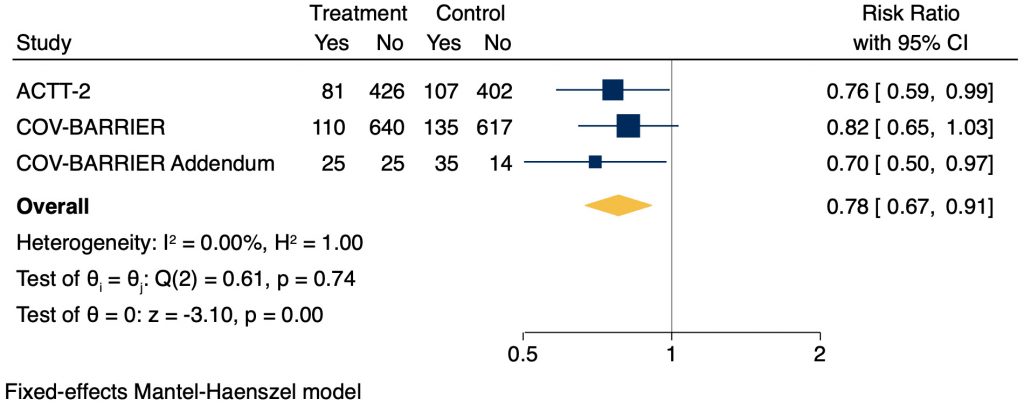
Adverse Events
Serious adverse events were significantly lower in patients receiving baricitinib as compared to placebo (RR 0.78, 95% CI 0.67 – 0.91; Figure 4). The most common serious adverse event reported was respiratory failure. Additionally, studies reported other adverse outcomes; the most common non-serious adverse events reported included hyperglycemia, anemia, lymphopenia, and acute kidney injury.
Additionally, the COV-BARRIER trial and its addendum showed a lower incidence of adverse events leading to treatment discontinuation (RR 0.80, 95% CI 0.60 – 1.08) or death (RR 0.5, 95% CI 0.28 – 0.89).7,8 There was no increased risk of infection with baricitinib as compared to placebo in any of the three RCTs. However, low frequency or delayed events may not have been captured in these studies.
Interpretation
In certain patients hospitalized with COVID-19, baricitinib reduces mortality. This effect is most notable in patients requiring supplemental oxygen as well as those requiring NIV or HFO; there is also a trend towards benefit in those on IMV or ECMO, but this is not statistically significant, and there is significant heterogeneity between the included trials.
In patients who did not require oxygen, results were not statistically significant with extremely wide confidence intervals. Our current understanding of the disease physiology and progression would suggest the baseline risk is sufficiently low that patient benefit would be negligible.
There has been some speculation that concomitant corticosteroid use may have an impact on patient outcomes. There was observed heterogeneity between the two trials (3 publications) in baseline corticosteroid use (79% in COV-BARRIER, 86% COV-BARRIER addendum, and 12% in ACTT-2) which may be a contributing factor in observed differences across the outcomes.6–8 However, the COV-BARRIER study conducted a subgroup analysis to examine the potential effect of steroid use on 28-day mortality; it found a mortality benefit in both subgroups with concomitant steroid use (hazard ratio (HR) 0.63, 95% CI 0.45 – 0.89) and without concomitant steroid use (HR 0.28, 95% CI 0.10 – 0.77).7,8 There are, however, other additional confounders between the trials including inclusion criteria, comorbidities, and geographic distribution (a potential surrogate for access to care and standard of care) which may also contribute to the observed differences.
Baricitinib also reduces progression to IMV or ECMO and may reduce progression to the combined outcome of HFO, NIV, IMV or death. Also, patients who received baricitinib were observed to have fewer serious adverse events as compared to those receiving placebo.
The recommended dose of baricitinib is 4 mg PO or NG daily (2 x 2 mg tablets) up to 14 days or until discharge from hospital. For patients with an eGFR 30-60 mL/min/1.73m2, the dose should be reduced to 2 mg PO or NG daily up to 14 days or until discharge from hospital.
Special Considerations with Pregnancy
Pregnant individuals are at increased risk of morbidity and mortality due to COVID-19. However, there is uncertainty around the benefits and harms of baricitinib for pregnant individuals with COVID-19.9 Pregnant patients were excluded from the ACTT-2, COV-BARRIER and addendum trials.6–8 Information on the use of baricitinib in pregnancy is limited to a single case report and there is insufficient data to assess the drug-associated risks for birth anomalies or pregnancy loss.10 Baricitinib is expected to cross the placenta based on molecular weight, and studies in animals have shown teratogenic effects at high doses, though no fetal effects were observed at exposures twice the human dose.11 Decisions about baricitinib administration in pregnancy need to be made through shared decision making with the patient, with consideration for the potential maternal benefit and theoretical fetal risks.
Special Considerations with Breastfeeding
Breastfeeding individuals were excluded from the ACTT-2, COV-BARRIER and addendum trials.2,4,5 It is unknown if baricitinib or metabolites are excreted in human milk, though transfer is expected due to molecular weight and animal data showing excretion of baricitinib in milk.11 A risk to newborn and infants cannot be excluded and baricitinib should not be routinely administered in breastfeeding individuals. Baricitinib can be administered if, following discussion of the potential risks and benefits, the patient is willing to stop breastfeeding during treatment. If breastfeeding is stopped during baricitinib treatment, patients with normal renal function should wait 72 hours after the last dose of baricitinib to begin breastfeeding.11
Special Considerations with Renal Impairment
Renal elimination is the principal mechanism for baricitinib clearance.11 Baricitinib for other indications is not recommended in patients with an eGFR <30 mL/min/1.73m2 and these patients were excluded from the ACTT-2, COV-BARRIER, and addendum trials.6–8,11 Baricitinib should not be used in these patients. In patients with eGFR 30-60 mL/min/1.73m2, the recommended daily dose is reduced to 2 mg, and this dose was also used in the ACTT-2, COV-BARRIER and addendum trials.6–8,11
Special Considerations with Concomitant Therapies
Dexamethasone is the standard of care in moderately and critically ill COVID-19 patients. In the COV-BARRIER and addendum trials, 79% and 86% of patients received concurrent corticosteroids, and a sub-group analysis in the COV-BARRIER trial showed mortality benefit in patients both with and without corticosteroids with no additional adverse events.4,5 Remdesivir is used in moderately ill patients and was administered alongside baricitinib in the ACTT-2 trial.2 Baricitinib should be administered alongside standard of care, which should include dexamethasone +/- remdesivir as indicated. Baricitinib can also be administered to patients who are unable to receive a corticosteroid because of a contraindication.
Tocilizumab and sarilumab are IL-6 inhibitors currently recommended in select moderately and critically ill COVID-19 patients. Patients on biologic therapies (including IL-6 inhibitors), were excluded from the ACTT-2, COV-BARRIER and addendum trials.2,4,5 IL-6 inhibitors and baricitinib are both immunosuppressants and there is limited information on the safety or efficacy of combining these agents outside of case reports. At this time combined use of IL-6 inhibitors and baricitinib is not recommended. There is no evidence directly comparing baricitinib and IL-6 inhibitors to inform a decision regarding which therapy is preferred. The decision to treat with baricitinib or an IL-6 inhibitor should be made based on clinical judgement and patient preference regarding availability, side effects and contraindications.
Practical Considerations
Patients who are pregnant or breastfeeding were not included in the above trials. Use in these populations should be determined through shared decision making with the patient to evaluate potential maternal benefits and theoretical fetal risks.
Additionally, the baricitinib dose should be reduced to 2 mg daily in patients with an eGFR 30-60 mL/min/1.73m2. It should not be used in patients with eGFR <30 mL/min/1.73m2.
Patients receiving baricitinib should have a complete blood count, creatinine function and liver enzymes monitored throughout the treatment duration.
Recommendations
Please see the section below entitled “Methods Used for this Scientific Brief” for a description of COVID-19 illness severity criteria.
Critically Ill Patients
Baricitinib 4 mg PO or NG daily (2 x 2 mg tablets) for 14 days or until discharge may be considered in patients receiving non-invasive or high-flow nasal oxygen, mechanical ventilation or ECMO who are on a recommended dose of dexamethasone therapy (or a dose-equivalent corticosteroid) or who have a contraindication to corticosteroid treatment.
The panel does not recommend combined use of baricitinib and IL-6 inhibitors due to absence of safety and efficacy evidence.
Moderately Ill Patients
Baricitinib 4 mg PO daily (2 x 2 mg tablets) for 14 days or until discharge may be considered in patients on supplemental oxygen who are on a recommended dose of dexamethasone therapy (or a dose-equivalent corticosteroid) or who have a contraindication to corticosteroid treatment.
The panel does not recommend combined use of baricitinib and IL-6 inhibitors due to absence of safety and efficacy evidence.
Mildly Ill Patients
Baricitinib is not recommended in mildly ill COVID-19 patients who do not require oxygen.
Methods Used for This Science Brief
We searched PubMed, Google Scholar, the COVID-19 Rapid Evidence Reviews, the Joanna Briggs Institute’s COVID-19 Special Collection, LitCovid in PubMed, the Oxford COVID-19 Evidence Service, the World Health Organization’s Global Literature on Coronavirus Disease, and other COVID-19 specific resources listed by the Guidelines International Network and the McMaster Health Forum. The search was last updated on October 15, 2021.
We conducted our meta-analyses using STATA Release 16.0 software (StataCorp LLC, College Station, TX) using a Mantel-Haenszel fixed-effects model.
*Update: The Ontario Science Table Drugs & Biologics Clinical Practice Guidelines Working Group has reviewed its definitions for COVID-19 disease severity. These definitions have always emphasized disease physiology and ventilatory support, as opposed to the setting in which care is delivered. Going forward, definitions for mild, moderate, and critically-ill disease will focus solely on the need for new oxygen or circulatory support and will not reference hospital admission status. These changes are reflected in the definitions below.
For therapeutic recommendations, we used the following definitions for severity:
Critically Ill
Patients requiring ventilatory and/or circulatory support, including high-flow nasal oxygen, NIV, invasive mechanical ventilation, or ECMO.
Moderately Ill
Patients newly requiring low-flow supplemental oxygen.
Mildly Ill
Patients who do not require new or additional supplemental oxygen from their baseline status.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: Consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033-1034. https://doi.org/10.1016/S0140-6736(20)30628-0
- Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The Lancet. 2020;395(10223). https://doi.org/10.1016/S0140-6736(20)30304-4
- Jorgensen SCJ, Tse CLY, Burry L, Dresser LD. Baricitinib: A review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacother J Hum Pharmacol Drug Ther. 2020;40(8):843-856. https://doi.org/10.1002/phar.2438
- Spinelli FR, Conti F, Gadina M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci Immunol. 2020;5(47). https://doi.org/10.1126/sciimmunol.abc5367
- Stebbing J, Phelan A, Griffin I, et al. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400-402. https://doi.org/10.1016/S1473-3099(20)30132-8
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. Published online December 11, 2020. https://doi.org/10.1056/NEJMoa2031994
- Marconi VC, Ramanan AV, Bono S de, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407-1418. https://doi.org/10.1016/S2213-2600(21)00331-3
- Ely EW, Ramanan AV, Kartman CE, et al. Baricitinib plus standard of care for hospitalized adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: Results of a randomized, placebo-controlled trial.; medRxiv. 2021:2021.10.11.21263897. https://doi.org/10.1101/2021.10.11.21263897
- Zambrano LD. Update: Characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status — United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69. https://doi.org/10.15585/mmwr.mm6944e3
- Costanzo G, Firinu D, Losa F, Deidda M, Barca MP, Del Giacco S. Baricitinib exposure during pregnancy in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2020;12. https://doi.org/10.1177/1759720X19899296
- Olumiant: Summary of product characteristics. Eli Lilly Nederland B.V; 2018. https://www.em
Author Contributions: AKH, SDJ, AMM and MP conceived the Science Brief. AKH, and SDJ wrote the first draft of the Science Brief. PB, SV and PJ performed the analyses. All authors revised the Science Brief critically for important intellectual content and approved the final version.
Citation: Hempel AK, Jevtic SD, Vandersluis S, et al. Baricitinib for hospitalized patients with COVID-19. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2022;3(53). https://doi.org/10.47326/ocsat.2022.03.53.1.0
Author Affiliations: The affiliations of the members of the Ontario COVID-19 Science Advisory Table can be found at https://covid19-sciencetable.ca/.
Declarations of Interest: The declarations of interest of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners can be found at https://covid19-sciencetable.ca/. The declarations of interest of external authors can be found under Additional Resources.
Copyright: 2022 Ontario COVID-19 Science Advisory Table. This is an open access document distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original work is properly cited.
The views and findings expressed in this Science Brief are those of the authors and do not necessarily reflect the views of all of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners.
