Heparin Anticoagulation for Hospitalized Patients with COVID-19
Authors:Stephanie Carlin, Andrew M. Morris, Zainab B. Abdurrahman, Jacob J. Bailey, Martin E. Betts, William Ciccotelli, Bradley J. Langford, Elizabeth Leung, Antonina Maltsev, Laveena Munshi, Ullanda Niel, Nathan M. Stall, Anupama Wadhwa, Peter Jüni, Menaka Pai, on behalf of the Ontario COVID-19 Science Advisory Table and the Drugs & Biologics Clinical Practice Guidelines Working Group
Key Message
Critically Ill Patients
Prophylactic dose low molecular weight or unfractionated heparin are recommended in critically ill patients hospitalized with COVID-19. These patients should not receive therapeutic dose anticoagulation unless they have a separate indication for this treatment. Therapeutic dose anticoagulation in this patient population does not reduce the need for organ support and may increase bleeding events as compared to prophylactic dose anticoagulation.
Moderately Ill Patients
Therapeutic dose low molecular weight or unfractionated heparin may be considered over prophylactic dose anticoagulation in moderately ill patients who are felt to be at low risk of bleeding. All other patients should receive prophylactic dose anticoagulation, unless they have a separate indication for therapeutic dose anticoagulation. Therapeutic dose anticoagulation may reduce the need for organ support (including the need for high-flow nasal oxygen) and appears to decrease thrombotic events in moderately ill patients compared to lower intensity anticoagulation. Its benefits on survival are unclear, and it may increase major bleeding events. Given the small absolute risk reduction for patient-important outcomes and the known harms, a strong recommendation for therapeutic dose anticoagulation in moderately ill patients cannot be made.
Mildly Ill Patients
There is insufficient evidence to make a recommendation around anticoagulation for mildly ill patients.
Lay Summary
Patients with COVID-19 may become ill because of a combination of viral infection and the inflammation produced by the body’s immune system in response to that infection. Inflammation can lead to blood clots which damage their lungs, heart, kidney, and other vital organs.
How Low Molecular Weight and Unfractionated Heparin Work
Unfractionated heparin is a blood thinner (also called an anticoagulant). Low molecular weight heparins (including drugs like dalteparin, enoxaparin and tinzaparin) are also anticoagulants, which come from unfractionated heparin that has been broken down into smaller pieces. These anticoagulant medications make it more difficult for blood to clot and can be given in different doses to prevent clots (prophylactic dose) or to treat clots (therapeutic dose). Additionally, there is some evidence from laboratory experiments not conducted in humans that unfractionated and low molecular weight heparins may reduce inflammation. Other laboratory experiments have shown that unfractionated heparin may prevent the SARS-CoV-2 virus from attacking cells.
How We Came to Our Recommendations
To understand low molecular weight and unfractionated heparin’s effects on patients admitted to hospital with COVID-19, we reviewed a multiplatform randomized controlled trial (RCT) – a study that coordinated three similar RCTs to provide information on a larger group of patients – and a multicentre RCT. The multiplatform and multicentre RCTs compared the effects of these medications for treatment (therapeutic doses) and prevention (prophylactic doses). These studies involved thousands of patients in many different countries. They looked at patients with moderate illness (who were on low flow oxygen) as well as patients with severe illness (who required breathing and/or circulation support, including high flow oxygen).
Our Recommendation for Patients Admitted to Hospital with COVID-19 with Critical Illness
Patients admitted to hospital with severe COVID-19 illness do not benefit from treatment dose anticoagulation, and may suffer harm from severe bleeding or the need for a blood transfusion. They should not receive therapeutic dose anticoagulation unless they need it for another medical condition. Prevention dose anticoagulation should be offered to these patients.
Our Recommendation for Patients Admitted to Hospital with COVID-19 with Moderate Illness
Therapeutic dose anticoagulation may be given to patients admitted to hospital with moderate COVID-19 illness who are felt to be at a low risk of bleeding, after a discussion between the patient and the treating physician. This treatment may reduce the number of days that these patients spend receiving “intensive care”, including high doses of oxygen, a ventilator, and/or medications that increase their blood pressure or help their heart pump. Therapeutic dose anticoagulation may reduce mortality, but may also increase the chance of severe bleeding or the need for a blood transfusion. For these reasons, therapeutic dose anticoagulation may not be appropriate for all patients. Prevention dose anticoagulation should be offered to all patients not on therapeutic dose, to reduce the chance that they will develop blood clots.
Our Recommendation for Patients with COVID-19 with Mild Illness
We do not have enough evidence to make a recommendation about anticoagulation for patients with mild COVID-19 illness, who are usually not admitted to hospital. In general, these patients do not receive anticoagulation unless they need it for another medical condition.
Side Effects of Anticoagulation
The most common side effect of anticoagulant medications is bleeding. Higher doses of anticoagulants are more likely to cause bleeding. In moderately ill patients admitted to hospital with COVID-19 who have a low risk of bleeding, the benefits of therapeutic dose anticoagulation may outweigh the risk of bleeding. Other patients may receive lower, preventative doses of anticoagulation.
In critically ill patients admitted to hospital with COVID-19, the benefits of therapeutic dose anticoagulation do not outweigh the risks of bleeding. These patients do not benefit from a higher dose of anticoagulant treatment. They should receive lower, prevention doses of anticoagulation.
Summary
Background
COVID-19-associated thrombosis, provoked by profound inflammation and viral-mediated effects on cells, has been reported since the early days of the pandemic.1 Despite the use of prophylactic dose anticoagulation, venous and arterial thromboses—including in situ pulmonary thrombosis, deep vein thrombosis, pulmonary embolism, myocardial infarction, stroke, intravenous catheter thrombosis, and limb ischemia—are common in patients with COVID-19. A meta-analysis of studies utilizing various thromboprophylactic strategies reported venous thromboembolism in 5.5% of admitted non-critically ill patients and 18.7% of critically ill patients when patients were not screened for venous thrombosis with imaging, and rates of 23% and 45.6% in non-critically ill and critically ill patients respectively when routine screening was used.2
Unfractionated and low molecular weight heparins are commonly used parenteral anticoagulants for the prevention and treatment of venous and arterial thromboembolism. Unfractionated heparin is a mixture of proteoglycans of various chain lengths which bind to anti-thrombin to inhibit coagulation factors. Low molecular weight heparins, including dalteparin, enoxaparin, and tinzaparin, are smaller chain length molecules fractionated from heparin which also bind to anti-thrombin and inhibit coagulation factors Xa and IIa.
In addition to their anticoagulant activity, heparins have anti-inflammatory and, possibly, antiviral activity. Anti-inflammatory effects occur through inhibition of neutrophil activation and function, interaction with vascular endothelium to prevent inflammatory mediator expression, prevention of vascular smooth muscle cell proliferation,3 and anticoagulant activity. In vitro studies have demonstrated that unfractionated heparin may bind to the SARS-CoV-2 spike protein and act as a competitive inhibitor for viral entry into the host cell.4 As such, it was hypothesized that an increased heparin dose in COVID-19 patients may improve outcomes through multiple mechanisms.
Questions
Does anticoagulation improve outcomes, such as mortality, need for mechanical ventilation, and organ support-free days for patients with COVID-19?
What are the indications for therapeutic dose and prophylactic dose heparin in moderately and critically ill patients?
What is the risk of major bleeding in moderately and critically ill patients?
Findings
Overview of Key Trials
Multiplatform Trial Including ATTACC, ACTIV-4a and REMAP-CAP
The randomized multiplatform trial including ATTACC, ACTIV-4a and REMAP-CAP compared therapeutic anticoagulation with usual care thromboprophylaxis (standard prophylactic or intermediate dose) in hospitalized COVID-19 patients. Enrollment was discontinued in the critically ill cohort on December 19, 2020, after an interim analysis showed that the statistical criterion for futility had been met. On January 22, 2021, enrollment in the non-critically ill cohort was discontinued on the advice of the data and safety monitoring boards after a planned analysis of data in accordance with the adaptive design. Papers describing both arms were published on August 4, 2021.5,6
The multiplatform RCT used a composite primary outcome combining in-hospital mortality and days free of organ support. This outcome was an ordinal scale combining in-hospital mortality (assigned a value of -1) and days free of organ support (invasive or non-invasive mechanical ventilation, high flow nasal oxygen, vasopressor therapy, or extracorporeal membrane oxygenation (ECMO)) to day 21 (worst score of -1 and best score of 21). For example, a patient who died at day 18 would be assigned a score of -1 and a patient who survived and no longer required organ support at day 15 would be assigned a score of 6. The multiplatform RCT also considered other patient-important outcomes such as survival to hospital discharge without thrombosis, bleeding, survival to hospital discharge, survival without organ support through 28 days, and survival without intubation; these were considered secondary outcomes.
The trial in the critically ill cohort enrolled 1,089 patients between April 21, 2020 and December 19, 2020 in 10 countries (United Kingdom, Ireland, Netherlands, Australia, New Zealand, Saudi Arabia, Canada, United States, Brazil and Mexico). The trial in the moderately ill cohort enrolled 2,219 patients between April 21, 2020 and January 22, 2021 in 9 countries (United States, Canada, United Kingdom, Brazil, Mexico, Nepal, Australia, Netherlands, and Spain). A substantial proportion of patients in this multiplatform trial did not receive other treatments with demonstrated evidence for benefit: only 60% of moderately ill patients were on corticosteroids (a therapy that is now nearly universally used in this population), 0.5% were on tocilizumab, and 36% were on remdesivir.
RAPID Trial
The randomized multicentre RAPID trial comparing therapeutic anticoagulation with thromboprophylaxis (prophylactic standard dose) in moderately ill hospitalized COVID-19 patients completed enrollment on April 12, 2021, and was published in preprint form on July 12, 2021.7
The RAPID trial’s primary outcome was a composite of intensive care unit (ICU) admission, non-invasive (bilevel or continuous positive airway pressure) or invasive mechanical ventilation, or death up to 28 days. Secondary outcomes included: all-cause death; the composite of any mechanical ventilation or all-cause death; ICU admission or all-cause death; ventilator-free days alive; organ support-free days alive; ICU-free days alive; hospital free days alive; renal replacement therapy; venous thromboembolism; arterial thromboembolism; and D-dimer level at 2 days ± 24 hours post-randomization.
The trial included 465 patients enrolled between May 29, 2020 and April 12, 2021 at 28 sites in 6 countries in 10 countries (Canada, Saudi Arabia, Brazil, United States, Ireland and United Arab Emirates). Patients were required to have D-dimer levels above the upper limit of normal (ULN) of the local hospital in the presence of an oxygen saturation ≤93% on room air, or ≥2 times the ULN irrespective of oxygen saturation. Compared to the multiplatform trial, more patients in the RAPID trial received other treatments with demonstrated evidence for benefit; 77% of patients were on steroids, 5% were on tocilizumab, and 13% were on remdesivir.
Outcomes in Moderately Ill Patients
The multiplatform trial showed that therapeutic-dose anticoagulation increased organ support-free days as compared to usual care thromboprophylaxis with a posterior probability of 99.0% (median adjusted odds ratio 1.29, 95% credible interval (CrI) 1.04 to 1.61). A total of 76.4% of patients in the usual care thromboprophylaxis group survived to hospital discharge without requiring organ support during the first 21 days, compared with 80.2% in the therapeutic-dose group. The median adjusted absolute improvement in organ support-free survival to hospital discharge was 4.6% with therapeutic anticoagulation (95% CrI 0.7 to 8.1). There was no statistically significant difference between groups in the secondary endpoint of survival to hospital discharge, with 91.8% of patients in the usual care thromboprophylaxis surviving to hospital discharge as compared to 92.7% in the therapeutic dose anticoagulation group (median odds ratio adjusted for age, sex, site, D-dimer stratum, and time epoch of enrollment 1.21, 95% CrI 0.87-1.68).
The RAPID trial showed no difference in the primary outcome (a composite of ICU admission, non-invasive or invasive mechanical ventilation, or death up to 28 days) between groups. This outcome occurred in 16.2% assigned to therapeutic heparin and 21.9% assigned to prophylactic heparin (odds ratio, 0.69; 95% confidence interval [CI], 0.43 to 1.10, p=0.12). The therapeutic heparin group had a lower incidence of death; death from any cause occurred in 4 patients (1.8%) with therapeutic heparin and in 18 (7.6%) with prophylactic heparin (odds ratio, 0.22; 95%-CI, 0.07 to 0.65).
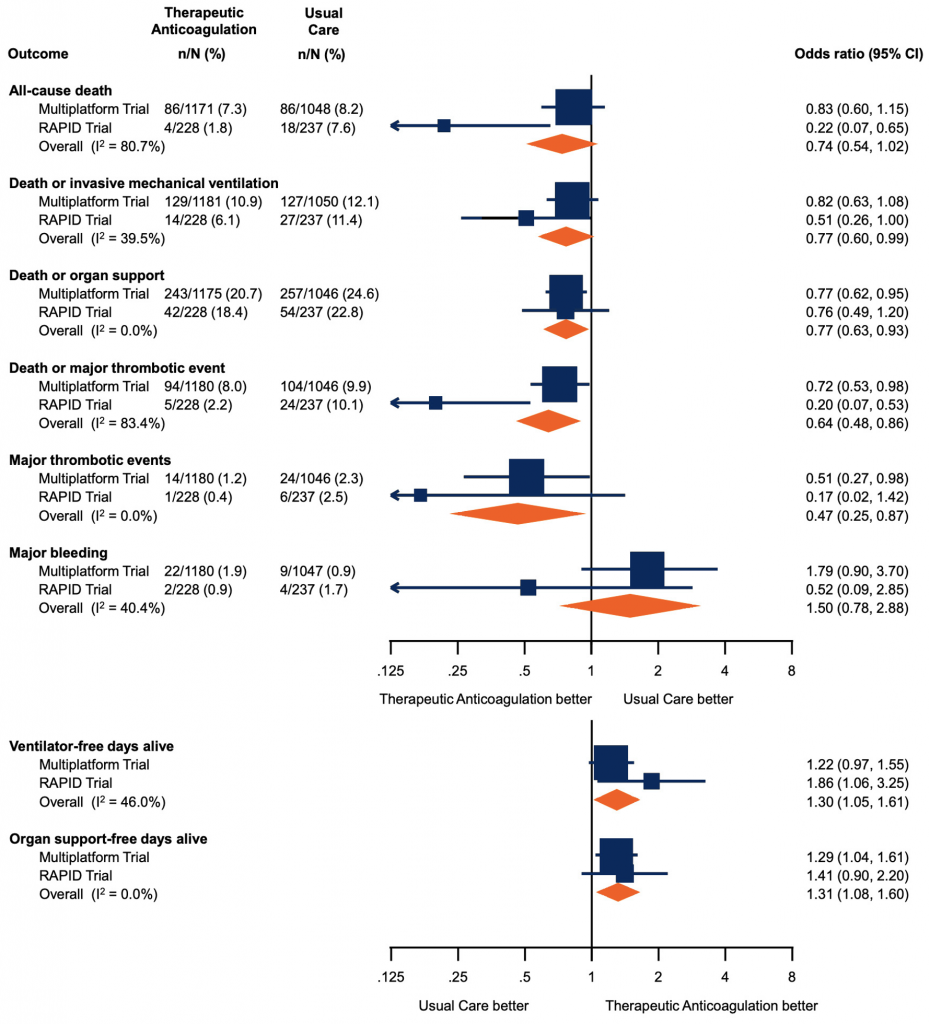
Forest plot presenting the odds ratios of efficacy and safety outcomes in moderately ill patients. Each square presents the results of an individual RCT with the size of squares proportional to the weights used in the meta-analysis and the horizontal lines indicating the 95% CIs. The solid vertical line at 1 represents the null effect (no effect on analyzed outcome) and the diamonds indicate the overall summary estimates from fixed-effect meta-analyses. Figure adapted from Sholzberg et al (2021).7 RCT, randomized controlled trial. CI, confidence interval.
Major bleeding occurred in 1.86% in the therapeutic anticoagulation arm as compared to 0.86% in the usual care thromboprophylaxis arm in the multiplatform trial, with a median adjusted proportional odds ratio for the secondary endpoint of freedom from major bleeding with therapeutic anticoagulation as compared to usual care thromboprophylaxis of 0.56 (95% CrI 0.27-1.11). Overt and symptomatic bleeding in a critical area or organ were rare with 9 events in the therapeutic anticoagulation group and 1 event in the usual care thromboprophylaxis group. There were 3 fatal bleeds in the therapeutic anticoagulation group and 1 fatal bleed in the usual care thromboprophylaxis group). Major bleeding occurred in 2 patients in the therapeutic heparin group and 4 patients in the prophylactic group (odds ratio 0.52; 95%-CI, 0.09 to 2.85) in the RAPID trial. There were no fatal bleeding events and no cases of intracranial hemorrhage in this trial.
A meta-analysis of RAPID and the multiplatform was performed, to evaluate safety and efficacy outcomes for moderately ill patients. There was no significant reduction in all-cause death (odds ratio, 0.74; 95%-CI, 0.54 to 1.02) with therapeutic heparin. There were significant reductions in the composite of death or invasive mechanical ventilation (odds ratio, 0.77; 95%-CI, 0.60 to 0.99), death or organ support (odds ratio, 0.77; 95%-CI, 0.63 to 0.93), death or major thrombotic event (odds ratio, 0.64; 95%-CI, 0.48 to 0.86), and major thrombotic events (odds ratio, 0.47; 95%-CI, 0.25 to 0.87). There were significant decreases in ventilator-free days alive (odds ratio, 1.30; 95%-CI 1.05 to 1.61) and organ support-free days alive (odds ratio 1.31, 95% 1.08 to 1.60) with therapeutic heparin. There was a non-significant increase in major bleeding.7
Outcomes in Critically Ill Patients
The multiplatform trial demonstrated that the median adjusted proportional odds ratio for the effect of therapeutic anticoagulation on organ support-free days was 0.87 (95% CrI 0.70-1.08), yielding a posterior probability of futility of 99.8% and a posterior probability of inferiority of 89.4%. The median value for organ support-free days was 3 (interquartile range -1, 16) in participants randomized to therapeutic anticoagulation as compared to 5 (interquartile range -1, 16) in patients assigned to usual care thromboprophylaxis.
Major bleeding occurred in 3.1% of patients in the therapeutic anticoagulation group as compared to 2.4% of patients in the usual care thromboprophylaxis group in the multiplatform trial, with a median adjusted proportional odds ratio for the safety endpoint of major bleeding with therapeutic anticoagulation as compared to usual care thromboprophylaxis of 1.46 (95% CrI 0.61-3.49).
Practical Considerations
Low molecular weight and unfractionated heparins are commonly used in the care of hospitalized patients. Protocols dealing with their use in specialized patient populations (e.g., renal failure, obesity) have been developed. In the Ontario context, we believe there are no specific concerns around cost, provincial supply, equity, feasibility, or acceptability associated with our recommendations.
Should Therapeutic Dose Anticoagulation Be Continued or Initiated in Critically Ill Patients Who Show Clinical Improvement, and Are Now Considered Moderately Ill?
We recommend continuation of prophylactic dose anticoagulation in critically ill patients who improve and can be classified as moderately ill, unless they develop another indication for therapeutic dose anticoagulation. This strategy was used in the multiplatform trial protocol, and is likely to be perceived as feasible and acceptable by clinicians. Moreover, the putative anti-inflammatory effects of therapeutic dose anticoagulation, potentially important to prevent progression to critical illness, are likely less important in patients who are clinically improving. At this time, there is insufficient evidence to support “stepping up” to therapeutic dose anticoagulation for patients who have clinically improved from critical to moderate illness.
Should Therapeutic Dose Anticoagulation Be Continued in Moderately Ill Patients Who Become Critically Ill?
We recommend continuation of therapeutic dose anticoagulation in moderately ill patients felt to be at low risk of bleeding who become critically ill, unless they develop a contraindication. This strategy was used in the multiplatform trial protocol, and is likely to be perceived as feasible and acceptable by clinicians. At this time, there is insufficient evidence to support “stepping down” to prophylactic dose anticoagulation at the time of transfer to a critical care setting.
Should We Use Standard or Intensified Prophylactic Dose Low Molecular Weight or Unfractionated Heparin?
There is insufficient evidence at this time to recommend an intensified prophylactic dose of anticoagulation (also called “intermediate dose”) in patients admitted to hospital with COVID-19. A multicentred RCT including 562 critically ill patients at 10 Iranian centres compared standard with intermediate dose anticoagulation (at a dose higher than standard prophylaxis, but lower than therapeutic anticoagulation) found no difference with respect to the composite endpoint of venous or arterial thrombosis, treatment with ECMO or 30 day mortality. Very low rates of venous thromboembolism were reported, however, and the study was underpowered.14
Should We Use Low Molecular Weight Heparin or Unfractionated Heparin?
Given the greater variability in anticoagulant response, increased laboratory monitoring requirements, and higher incidence of heparin induced thrombocytopenia with unfractionated heparin as compared to low molecular weight heparin, low molecular weight heparin is generally preferred over unfractionated heparin.12 Intravenous unfractionated heparin may, however, be considered in patients with severe renal dysfunction (creatinine clearance less than 30 mL/min), who would benefit from a shorter acting anticoagulant (e.g., those with high bleeding risk, those who require brief periods of anticoagulant interruption), or in situations where subcutaneous medication absorption may be reduced or erratic (i.e., anasarca).
Special Considerations with Pregnancy
While pregnant patients were excluded from the ACTIV-4a and RAPID trials (pregnancy not specified among exclusion criteria in ATTACC and REMAP-CAP trials), pregnant patients are at increased risk of morbidity and mortality due to COVID-19.15 Low molecular weight and unfractionated heparins, due to their molecular size, do not cross the placenta.16Given the extensive experience and safety associated with these anticoagulants in pregnancy, we believe that the recommendations outlined in this document should apply to pregnant patients.
Special Considerations with Breastfeeding
Unfractionated heparin, due to its molecular size, is not found in breastmilk. Small amounts of low molecular weight heparin may pass into breast milk, however, given their low bioavailability, do not result in an anticoagulant effect in the baby. Given the extensive experience and safety associated with both of these anticoagulants in lactation, we believe that the recommendations outlined in this document should apply to lactating patients.
Recommendations
Please see the below section entitled “Methods Used for this Scientific Brief” for a description of COVID-19 illness severity criteria.
Critically Ill Patients
Prophylactic dose low molecular weight or unfractionated heparin are recommended in critically ill patients hospitalized with COVID-19. These patients should not receive therapeutic dose anticoagulation unless they have a separate indication for this treatment. Therapeutic dose anticoagulation in this patient population does not reduce the need for organ support and may increase bleeding events as compared to prophylactic dose anticoagulation.
The panel did not specifically review the indirect evidence for the use of prophylactic dose anticoagulation versus no anticoagulation in critically ill patients with COVID-19. Risk assessment models to estimate thrombotic risk in critically ill patients exist, but none have been specifically validated in patients with COVID-19.8,9 However critically ill patients with COVID-19 are at higher risk of venous thromboembolism by virtue of having an acute infectious disease, organ failure, and may be considered to have a hypercoagulable state (given their high incidence of clinically evident thrombosis). Many critically ill patients also have additional risk factors for thrombosis, including older age and immobility. For this reason, all critically ill patients are considered high risk for thrombosis, even after routine prophylactic anticoagulation; pharmacologic venous thromboembolism prophylaxis is accepted as standard of care, unless there are clear bleeding contraindications.10,11 For this reason, prophylactic dose anticoagulation is likely to be perceived as feasible and acceptable by clinicians and patients, versus no anticoagulation. The rates of major bleeding in critically ill patients on prophylactic dose anticoagulation is approximately 5%; this is comparable to the findings of the multiplatform trial.12
Moderately Ill Patients
Therapeutic dose low molecular weight or unfractionated heparin may be considered over prophylactic dose anticoagulation in moderately ill patients who are felt to be at low risk of bleeding. All other patients should receive prophylactic dose anticoagulation, unless they have a separate indication for therapeutic dose anticoagulation. Therapeutic dose anticoagulation may reduce the need for organ support (including the need for high-flow nasal oxygen) and appears to decrease thrombotic events in moderately ill patients compared to lower intensity anticoagulation. Its benefits on survival are unclear, and it may increase major bleeding events. Given the small absolute risk reduction for patient-important outcomes and the known harms, a strong recommendation for therapeutic dose anticoagulation in moderately ill patients cannot be made at this time.
Risk assessment models to estimate bleeding risk in hospitalized patients exist, but none have been specifically validated in patients with COVID-19.13 The cumulative incidence of major in-hospital bleeding within 14 days of admission in acutely ill hospitalized medical patients is approximately 1.2%. The panel defined patients at higher risk of bleeding as those who would have been excluded from the multiplatform trial: intracranial surgery or stroke within 3 months, history of intracerebral arteriovenous malformation, cerebral aneurysm or mass lesion of the central nervous system, intracranial malignancy, history of bleeding diatheses such as hemophilia, history of gastrointestinal bleeding within 3 months, thrombolysis within 1 week, epidural or spinal catheter, major surgery within 2 weeks, uncontrolled hypertension with systolic blood pressure over 200 mmHg or diastolic blood pressure over 120 mmHg, platelet count less than 50 x 10^9/L, INR over 2, baseline aPTT over 50 seconds, hemoglobin less than 80 g/L, acute or subacute bacterial endocarditis, or concurrent use of dual antiplatelet therapy.
A minority (⅓) of the guideline panel voted against the use of therapeutic dose anticoagulation versus prophylactic dose anticoagulation in moderately ill patients at low risk of bleeding. The majority (⅔) of the guideline panel voted for a conditional recommendation for therapeutic dose anticoagulation versus prophylactic dose anticoagulation in moderately ill patients at low risk of bleeding. The panel’s ultimate recommendation is based on the perceived importance of both mortality and organ support free days to patients (in particular, avoiding intubation and possible transfer to another institution if a higher level of care is needed). There was a small absolute benefit for these outcomes, and the panel noted that The RAPID trial suggested that though therapeutic dose anticoagulation may reduce all-cause death, this benefit was not sustained when data from the RAPID trial and the multiplatform trial were meta-analyzed. They also noted that both the multiplatform trial’s and the RAPID trial’s definition of organ support free days included avoiding respiratory support via high-flow nasal cannula, an outcome perceived to be less important to many patients. The panel’s ultimate recommendation is also based on the perceived importance of survival to hospital discharge to patients, an outcome that showed minimal absolute benefit in the multiplatform trial.
It should be noted that the available evidence for anticoagulation reflects patients who largely did not receive other evidence-based therapies for COVID-19 such as tocilizumab, and remdesivir. The panel hypothesized that with improved, evidence-based therapy, the small absolute benefits of anticoagulation may be less pronounced, while its bleeding risks would remain the same. The panel also notes that the benefits of therapeutic anticoagulation may have been diluted in the multiplatform trial, and the harms of prophylactic anticoagulation may have been overstated, as 26.5% (227/855) of patients in the usual care group received intermediate dose thromboprophylaxis.
The panel did not specifically review the indirect evidence for the use of prophylactic dose anticoagulation versus no anticoagulation in moderately ill patients with COVID-19. Risk assessment models to estimate thrombotic risk in hospitalized patients exist, but none have been specifically validated in patients with COVID-19.8,9 Patients with COVID-19 are at higher risk of venous thromboembolism by virtue of having an acute infectious disease, and may be considered to have a hypercoagulable state (given their high incidence of clinically evident thrombosis). Many moderately ill patients also have additional risk factors for thrombosis, including age, immobility, and respiratory failure. For this reason, prophylactic dose anticoagulation is likely to be perceived as feasible and acceptable by clinicians and patients, versus no anticoagulation.
Mildly Ill Patients
There is currently insufficient evidence to make a recommendation around anticoagulation for mildly ill patients.
Methods Used for This Science Brief
We searched PubMed, Google Scholar, the COVID-19 Rapid Evidence Reviews, the Joanna Briggs Institute’s COVID-19 Special Collection, LitCovid in PubMed, the Oxford COVID-19 Evidence Service, the World Health Organization’s Global Literature on Coronavirus Disease, and other COVID-19 specific resources listed by the Guidelines International Networkand the McMaster Health Forum. In addition, we retrieved reports citing relevant articles through Google Scholar and reviewed references from identified articles for additional studies. The search was last updated on August 20, 2021.
For therapeutic recommendations, we used the following definitions for severity:
Patients requiring ventilatory and/or circulatory support, including high-flow nasal oxygen, non-invasive ventilation, invasive mechanical ventilation, or ECMO. These patients are usually managed in an intensive care setting.
Patients newly requiring low-flow supplemental oxygen. These patients are usually managed in hospital wards.
Patients who do not require new or additional supplemental oxygen from their baseline status, intravenous fluids, or other physiological support. These patients are usually managed in an ambulatory/outpatient setting.
