Remdesivir for Hospitalized Patients with COVID-19
Authors:Andrew M. Morris, Peter Jüni, Ayodele Odutayo, Pavlos Bobos, Nisha Andany, Kali Barrett, Martin Betts, Andrew Healey, Bradley Langford, Antonina Maltsev, Katherine J. Miller, Justin Morgenstern, Laveena Munshi, Fahad Razak, Nathan M. Stall, Menaka Pai on behalf of the Drugs & Biologics Clinical Practice Guidelines Working Group and the Ontario COVID-19 Science Advisory Table
Key Message
Remdesivir, a direct-acting antiviral agent, may reduce mortality and progression to mechanical ventilation in moderately ill patients hospitalized with COVID-19 on supplemental low-flow oxygen. The benefits of remdesivir for critically ill patients requiring supplemental oxygen via high-flow nasal cannula or mask, or non-invasive mechanical ventilation, is uncertain. Remdesivir does not benefit and may harm critically ill patients already receiving mechanical ventilation or requiring extra-corporeal membrane oxygenation (ECMO), and it does not provide substantial benefit for hospitalized patients who do not require supplemental oxygen. Remdesivir appears to have comparable effects when used for 5 days or 10 days, and does not appear to be associated with significant adverse effects.
Remdesivir is recommended in moderately ill hospitalized patients with COVID-19 requiring supplemental oxygen (Figure 1). Remdesivir may be considered for patients requiring oxygen supplementation via high-flow nasal cannula or mask, or non-invasive mechanical ventilation. It should not be used in critically ill patients on mechanical ventilation or those receiving ECMO. Remdesivir should not be used in patients who do not require supplemental oxygen.
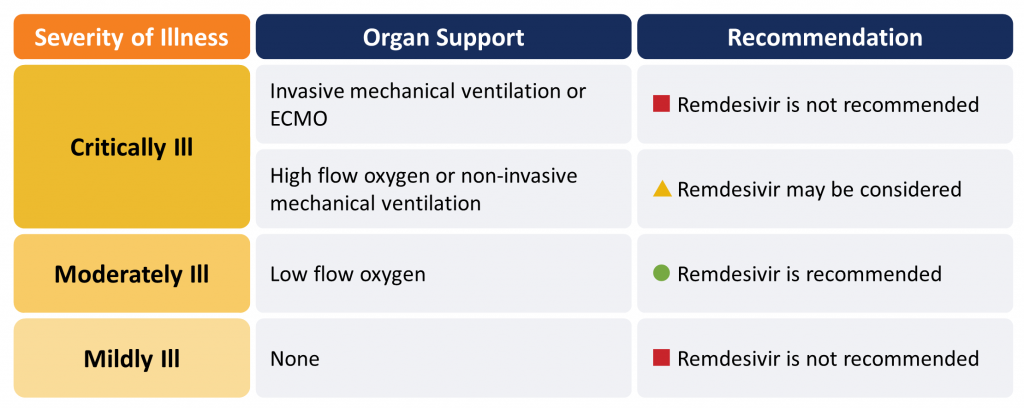
Please see the section below entitled “Methods Used for this Scientific Brief” for a description of COVID-19 illness severity criteria.
Lay Summary
Remdesivir Might Save Lives in Hospitalized COVID-19 Patients, with Moderate Illness
Patients with COVID-19 are ill because of a combination of viral infection and the inflammation that their body mounts to respond to that infection. Inflammation can compromise their breathing and damage their lungs, heart, kidney, and other vital organs.
Remdesivir is an antiviral medication approved by Health Canada for patients with severe symptoms of COVID-19. It is approved for adults and children aged 12 years and older who weigh at least 40 kg. Remdesivir might improve survival and might prevent hospitalized patients with moderate severity disease from needing mechanical ventilation (a breathing tube). As a COVID-19 treatment, it should only be considered in hospitalized patients on supplemental oxygen whom doctors determine to be “moderately” ill with COVID-19. It is not appropriate for patients who are either mildly ill with COVID-19 or critically ill with COVID-19 and requiring invasive ventilation.
Importantly, most remdesivir studies were performed before corticosteroids, such as dexamethasone, were widely used to treat COVID-19 patients. Since corticosteroids are effective treatments when prescribed for COVID-19, it is unclear from the remdesivir trial data we reviewed whether remdesivir provides additional benefit.
How Remdesivir Works
Remdesivir is an antiviral drug that is given intravenously. It works by blocking certain viruses from reproducing, including SARS-CoV-2, the virus that causes COVID-19.
How We Came to Our Recommendations
To understand remdesivir’s effect on patients hospitalized with COVID-19, we reviewed four randomized controlled trials (RCTs) from around the world that compared remdesivir to placebo or to routine care alone. Two of these trials were very large. The results of the trials varied, but when taken together, they showed that remdesivir might decrease death and the need for mechanical ventilation and speeds up recovery when given to moderately ill hospitalized patients with COVID-19 on supplemental oxygen.
COVID-19 patients taking remdesivir in these studies very rarely had side-effects; in fact, they were less likely to suffer adverse events than patients in the placebo or routine care groups.
Remdesivir Availability in Ontario
There is a limited supply of remdesivir in Ontario. For this reason, doctors should be careful about using the drug only when it is needed.
Our Recommendation for Using Remdesivir as a Treatment for COVID-19
We recommend that patients hospitalized with COVID-19 should receive remdesivir if they have moderate illness requiring supplemental oxygen.
Remdesivir may be considered for patients who need more oxygen support than supplemental oxygenation by nasal prongs, but do not require mechanical ventilation. In these patients, it is uncertain whether remdesivir is helpful.
At this time, we do not recommend remdesivir for patients who are mildly ill with COVID-19.
We also do not recommend remdesivir for patients who are critically ill, requiring mechanical ventilation or ECMO.
Summary
Background
Remdesivir is an antiviral drug that has been shown to be effective against the SARS-CoV-2 virus in animal models and laboratory studies.1,2 There are currently four RCTs comparing remdesivir to placebo or usual care,3–6 and another RCT that compared two different durations of remdesivir without a control group.7
In July 2020, Health Canada approved the use of remdesivir for the treatment of hospitalized adults and children (aged 12 years and over with body weights of at least 40 kg) with COVID-19 pneumonia requiring supplemental oxygen.
Questions
Does remdesivir improve patient outcomes such as mortality, need for mechanical ventilation, requirement for organ support, intensive care unit (ICU) length of stay, and hospital length of stay for patients hospitalized with COVID-19?
Which patients hospitalized with COVID-19 will receive the most benefit from remdesivir?
What is the recommended duration of therapy for remdesivir?
Findings
As of March 1, 2021, four RCTs have been published in peer-reviewed journals, studying the effect of remdesivir compared with placebo or usual care in a total of 7,334 patients hospitalized with COVID-19. Disease severity for the patients with COVID-19 enrolled in these trials ranged from mild (i.e., not requiring supplemental oxygen) to critically ill. A fifth trial compared 5 days of remdesivir therapy to 10 days in patients hospitalized with COVID-19 requiring supplemental oxygen or ventilatory support at randomization, but who were not mechanically ventilated or on ECMO.
It is important to note that all of these trials were completed before the emergence of strong evidence in support of corticosteroids and IL-6 inhibitors like tocilizumab for the treatment of COVID-19.
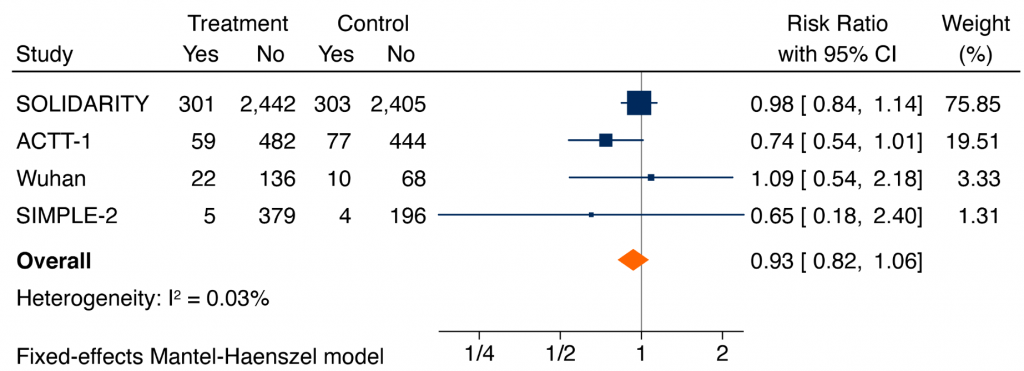
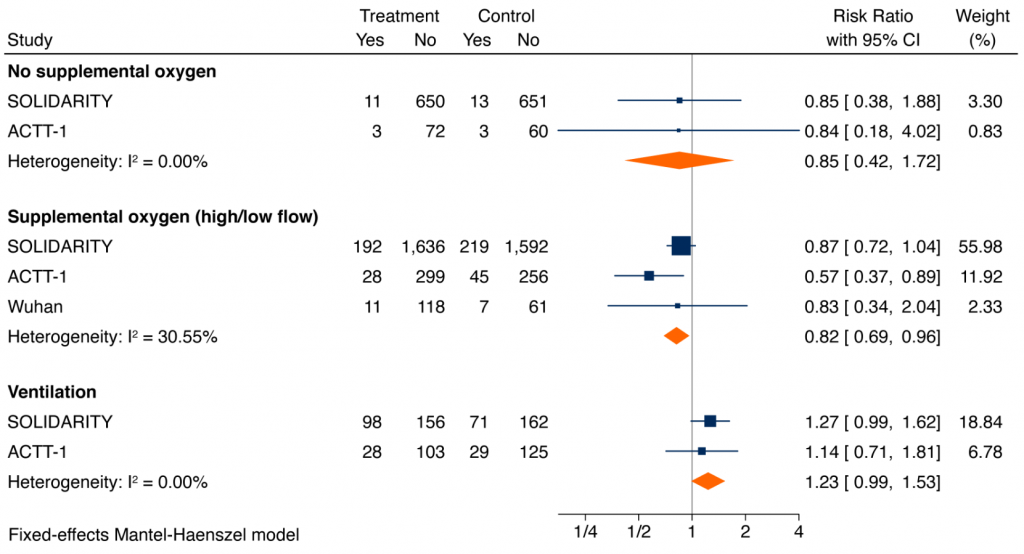
A meta-analysis of RCTs of the effect of remdesivir on all-cause mortality at 14-28 days in hospitalized patients with COVID-19 irrespective of their severity of illness was inconclusive (pooled risk ratio (RR) 0.93, 95% confidence interval (CI) 0.82 to 1.06). However, for those on supplemental oxygen and not ventilated at baseline, the pooled RR is 0.82 (95% CI 0.69 to 0.96), corresponding to an absolute risk reduction of 2.3% and a number-needed-to-treat to save one life of 44. For hospitalized patients on no supplemental oxygen, the pooled RR was 0.85 (95% CI 0.42 to 1.72), similar to patients on supplemental oxygen, but with an absolute risk reduction of 0.3%, and a number-needed-to-treat to save one life of 334. The RR for critically ill patients on mechanical ventilation or high-flow oxygen was 1.23 (95% CI 0.99 to 1.53). The difference in treatment effects in critically ill patients on mechanical ventilation or high-flow oxygen as compared with remaining patients was statistically significant (p-value for interaction = 0.004).
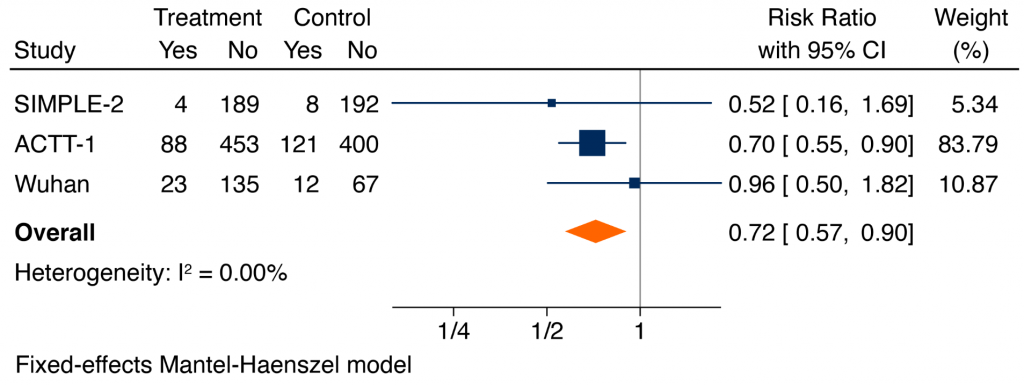
Remdesivir reduced the composite of mechanical ventilation or death at day 28, with a RR of 0.71 (95% CI 0.57 to 0.88).
In the ACTT-1 trial, the median time to recovery was 2 days shorter for those on supplemental oxygen via nasal prongs at baseline with remdesivir as compared with placebo (7 days versus 9 days; RR 1.45, 95% CI 1.18 to 1.79). In two smaller RCTs, remdesivir shortened the time to clinical improvement, with a pooled hazard ratio of 1.17 (95% CI 1.02 to 1.34).
Remdesivir versus placebo may reduce hospital length of stay, with a mean reduction of 5 days (95% CI 2.3 to 7.7 days). Because the SOLIDARITY trial required patients to remain in hospital to complete a full course of treatment, the study design biased the trial towards no effect on hospital length of stay.
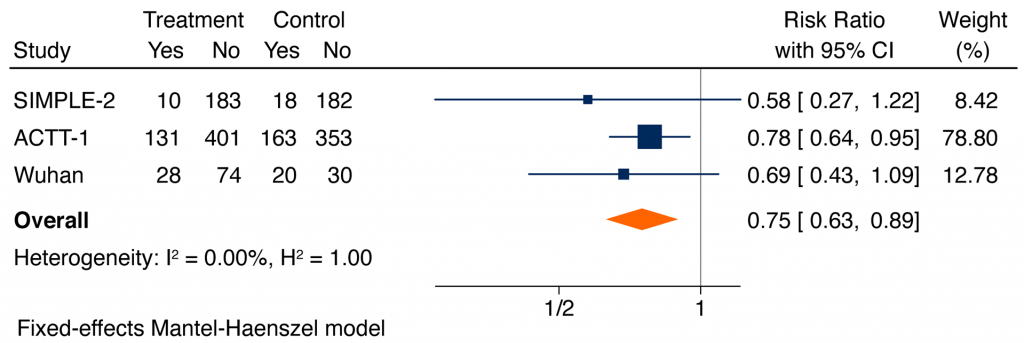
Based on all available data, serious adverse events were significantly lower in patients receiving remdesivir as compared with placebo or usual care (RR 0.75, 95% CI 0.63 to 0.89). The most common reported serious adverse events were respiratory failure, and the most common non-serious adverse events reported were decreased renal function, anemia, lymphopenia, respiratory failure, fever, and hyperglycemia.
There are two published RCTs that address the duration of therapy with remdesivir. One trial compared remdesivir 5 days versus 10 days in 397 patients with mild to moderate illness, whereas the other trial compared remdesivir 5 days with 10 days or usual care in a 1:1:1 ratio in 596 patients (402 in the two remdesivir arms) with moderate to critical illness. The different dosing regimens had no effect on 28-day mortality (RR 0.87, 95% CI 0.53 to 1.40) or on the 28-day incidence of clinical improvement (RR 1.06, 95% CI 0.87 to 1.30). There were fewer adverse events with the 5-day regimen (RR 0.65, 95% CI 0.48 to 0.89).
Practical Considerations
There is a limited supply of remdesivir in Ontario. For this reason, clinicians should be selective about using the drug only when it is needed. In Ontario, remdesivir is distributed to hospitals through the Canadian Pharmaceutical Distribution Network (CPDN). In order to maximize equity across the province and maintain a stable supply, clinicians are encouraged to follow evidence-based principles in prescribing remdesivir. Some strategies for tocilizumab are also applicable to remdesivir.8
Recommendations
Please see the section below entitled “Methods Used for this Scientific Brief” for a description of COVID-19 illness severity criteria.
Critically Ill Patients (requiring high-flow oxygen, ventilation or ECMO):
Remdesivir is not recommended for critically ill patients with COVID-19 receiving mechanical ventilation or ECMO.
In patients requiring high-flow oxygen (i.e., oxygen by mask, oxygen by high-flow nasal cannula, or non-invasive mechanical ventilation), remdesivir 200 mg day 1 then 100 mg daily for 4 days may be considered for suspected or confirmed COVID-19.
Moderately Ill Patients (requiring low-flow supplemental oxygen):
Remdesivir 200 mg day 1 then 100 mg daily for 4 days is recommended for patients who are moderately ill with suspected or confirmed COVID-19.
Mildly Ill Patients (not requiring new or additional oxygen):
Remdesivir is not recommended for patients who are mildly ill with suspected or confirmed COVID-19.