Rollout Strategy for the Pfizer-BioNTech COVID-19 Vaccine in Ontario
Authors:Peter Jüni, Ashleigh R. Tuite, Isaac I. Bogoch, Adalsteinn D. Brown, Yoojin Choi, Bruno R. da Costa, Gerald A. Evans, David N. Fisman, Antonina Maltsev, Douglas G. Manuel, Sharmistha Mishra, Samira Mubareka, Fahad Razak, Arthur S. Slutsky, Nathan M. Stall, Tania Watts, Allison McGeer on behalf of the Ontario COVID-19 Science Advisory Table
Key Message
Administering Pfizer-BioNTech’s COVID-19vaccine during the early stage of the vaccine rollout (January/February 2021) to as many individuals as possible would prevent more COVID-19 cases in Ontario as compared to reserving half of the initial allotments as second booster doses (Figure 1).
On-label use of the vaccine with the administration of two doses is important, as the second dose significantly boosts the immune response and results in a substantial increase in neutralizing antibodies. However, using 100% of the initial allotments immediately to vaccinate as many individuals as possible does not preclude on-label use with two doses, even though the interval between first and second booster dose may become longer than 21 days.
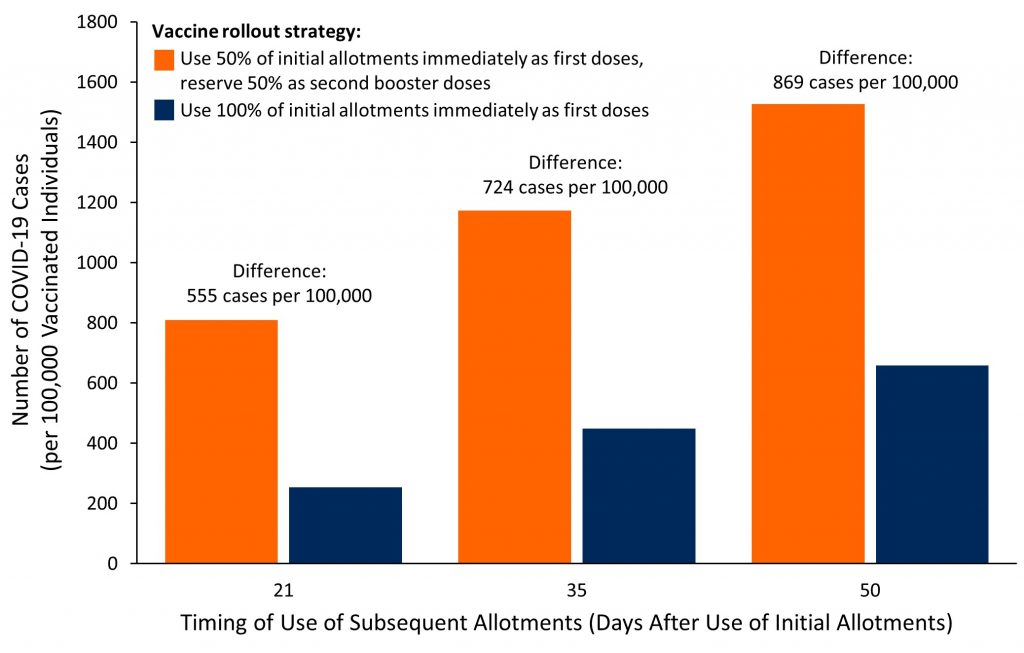
Figure 1. Number of Projected COVID-19 Cases per 100,000 Vaccinated Individuals Within 50 Days (Days 11 to 60 of Use of Initial Allotments), by Vaccine Rollout Strategy and Timing of Use of Subsequent Allotments Bar graph presenting the projected number of cases per 100,000 vaccinated individuals at high risk of infection vaccinated with Pfizer BioNTech’s COVID-19 vaccine, in Toronto, Ontario. The theoretical estimated number of cases without a vaccine was 2,800 per 100,000 individuals for the duration of 50 days (from day 11 after administration of the first dose of the vaccine to day 60). The following assumptions were used: the rate of daily reported cases of 28 per 100,000 individuals observed in Toronto’s communities with the highest proportion of essential workers currently reported (Mishra S, personal communication), which was used for illustrative purposes, will remain stable over the entire period of 50 days; the percentage of unreported COVID-19 cases is 50%; the efficacy of the first dose of Pfizer-BioNTech’s COVID-19 vaccine is 85% up to day 27, and decreases to 70% thereafter. The intervals of 35 and 50 days were chosen arbitrarily for illustrative purposes. See Table 1 for calculations of the efficacy of the first dose of the vaccine, Table 2 for the estimated effectiveness of the two rollout strategies by day of use of subsequent allotments, and Figure 2 for predicted cumulative incidence over time by rollout strategy and day of use of subsequent allotments. Estimates only reflect direct effects of the vaccine on vaccinated individuals but do not reflect indirect effects on unvaccinated individuals through decreased transmission.
Summary
Background
Ontario began administering the first doses of Pfizer-BioNTech’s COVID-19 vaccine on December 14, 2020, with an initial strategy to reserve 50% of the vaccine supply for administration as second booster doses 21 days later, as approved by Health Canada and other regulatory agencies across the world, which closely mirrors the design of the Phase III clinical trial.
Questions
What is the clinical efficacy of the first dose of Pfizer-BioNTech’s COVID-19 vaccine found in the Phase III clinical trial?
How effective is the initial strategy of reserving 50% of the vaccine supply to be administered as second booster doses, as compared with immediately administering 100% of available doses as first doses to twice as many people during the early stage of the vaccine rollout?
What are the implications of delaying the administration of the second booster dose of the vaccine later than 21 days after the first dose in case of delays related to the supply chain?
Findings
The first dose of Pfizer-BioNTech’s COVID-19 vaccine has an estimated efficacy of 85% (95% confidence interval (CI) 66 to 93%) in preventing COVID-19 cases as compared with a placebo, between 11 and 21 days after vaccination. A strategy of using 100% of initial allotments immediately during the early stage of the vaccine rollout (January/February 2021) is more effective in preventing COVID-19 cases as compared with the initial strategy of reserving 50% of initial allotments as second booster doses.
Interpretation
Administering Pfizer-BioNTech’s COVID-19 vaccine during the early stage of the vaccine rollout (January/February 2021) to as many individuals as possible would decrease the overall number of COVID-19 cases in Ontario, as compared to reserving half of the initial allotments as second booster doses.
On-label use of the vaccine with the administration of two doses is important, as the second dose significantly boosts the immune response and results in a substantial increase in neutralizing antibodies. It may also be important in assuring the overall durability of the immune response. Using 100% of the initial allotments immediately to vaccinate as many individuals as possible does not preclude on-label use with two doses, with the second booster dose administered from a subsequent allotment as soon as it becomes available.
These conclusions also apply to Moderna’s COVID-19 vaccine, which has a similar efficacy of the first dose.
Background
Health Canada approved the use of Pfizer-BioNTech’s COVID-19 vaccine, BNT162b2, on December 9, 2020. The approval was based on the results from Polack and colleagues’ Phase III clinical trial involving approximately 43,000 individuals recruited in the United States, Argentina, Brazil, South Africa, Germany, and Turkey.1
The trial established that the vaccine, when administered twice at an interval of 21 days, has an efficacy of 95% in preventing COVID-19 cases (95% CI 90% to 97%). This means that for every 100 cases of symptomatic COVID-19 in the unvaccinated control group, only 5 cases of symptomatic COVID-19 were observed in the vaccine group. With the available data, it is currently unclear whether Pfizer-BioNTech’s COVID-19 vaccine also reduces the risk of asymptomatic cases of SARS-CoV-2 infection, and the risk of transmission to others.
In the Phase III trial, the vaccine was administered via two intramuscular injections of 30 mg each, 21 days apart.1 The approved vaccination schedule for Pfizer-BioNTech’s COVID-19 vaccine is identical to the schedule used in the trial, with the interval between the first and second booster dose recommended to range between 19 and 28 days by Canada’s National Advisory Committee on Immunization (NACI).2
Ontario began administering the first doses of the vaccine on December 14, 2020, with an initial strategy of reserving 50% of the vaccine supply for administration as second booster doses 21 days later, closely mirroring the design of the clinical trial.1
On-label use of the vaccine with the administration of two doses of 30 mg each is important, as the second dose significantly boosts the immune response and results in a substantial increase in neutralizing antibodies.3
However, there may be upfront public health benefits in administering Pfizer-BioNTech’s COVID-19 vaccine during the early stage of the vaccine rollout to as many individuals as possible without reserving half of the initial allotments as second booster doses if the current Ontario vaccine supply chain remains relatively stable, and variation in supply will not, or will only moderately, delay the timing of the booster dose.
Questions
What is the clinical efficacy of the first dose of Pfizer-BioNTech’s COVID-19 vaccine found in the Phase III clinical trial?
How effective is the initial strategy of reserving 50% of the vaccine supply to be administered as second booster doses, as compared with immediately administering 100% of available doses as first doses to twice as many people during the early stage of the vaccine rollout?
What are the implications of delaying the administration of the second booster dose of the vaccine later than 21 days after the first dose in case of delays related to the supply chain?
Findings
Table 1 presents an analysis of COVID-19 cases that occurred during days 11 to 21 following the receipt of the first dose of the vaccine in the Phase III trial,1 which indicates an estimated efficacy of 85% for the first dose in preventing COVID-19 cases as compared with placebo. This estimate was not stated in the published report.1 This means that for every 100 cases of symptomatic COVID-19 in the unvaccinated control group, only 15 cases of symptomatic COVID-19 would be observed during days 11 to 21 after the first dose of the vaccine.
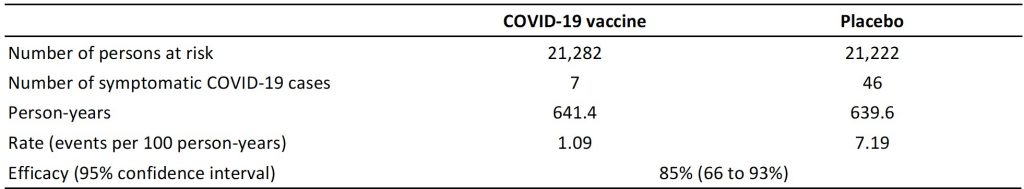
Table presenting the efficacy of the first dose of the Pfizer-BioNTech’s COVID-19 vaccine when the vaccine became efficacious, but before the second booster dose was administered. Efficacy was estimated from COVID-19 cases that occurred from days 11 to 21 after administration of the first dose of the vaccine or placebo. Number of events were manually and electronically extracted from time-to-event curves presented in Figure 3 by Polack et al.,1 with numbers of persons at risk and person-years estimated from information provided by Polack et al. and in the United States Food and Drug Administration’s briefing document on Pfizer-BioNTech’s COVID-19 vaccine.1,4
It is unclear how long the efficacy of 85% established up to day 21 will last, but a sudden change in the slope of the time-to-event curve appears unlikely. In addition, data on antibody responses to a related vaccine, which is based on the same technology but targets a different part of the SARS-CoV-2 spike protein, suggest no significant decrease in antibody levels against SARS-CoV-2 after a single injection with 60 mg between day 21 and day 43 after injection.5
Table 2 shows the estimated effectiveness of the initial strategy of reserving 50% of initial allotments as second booster doses as compared to an alternate strategy of using 100% of initial allotments immediately in preventing symptomatic COVID-19 cases. The alternate rollout strategy would allow for the vaccination of twice as many individuals during the early stage of the vaccine rollout, albeit with the potential of delaying the second booster dose in case of supply chain issues. As it is unclear how long the efficacy of 85% of the first dose will last, the model assumed that the efficacy of the first dose would decrease to 70% from day 28 onwards until 7 days after administering the second booster dose.
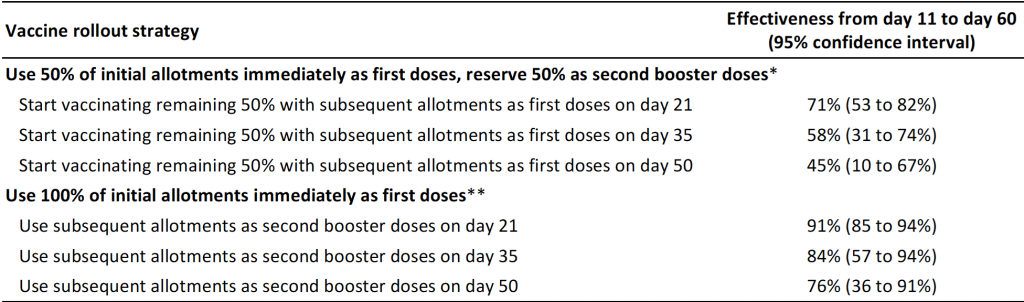
Table presenting the effectiveness of the initial rollout strategy of reserving 50% of initial allotments of Pfizer-BioNTech’s COVID-19 vaccine as second booster doses, as compared with an alternate rollout strategy of using 100% of initial allotments immediately. Effectiveness was estimated for the population included by Polack et al.1 using participants allocated to placebo as the control group. An effectiveness of 84% means, for example, that for every 100 cases of symptomatic COVID-19 in an unvaccinated population, only 16 cases of symptomatic COVID-19 would be observed during days 11 to 60 with the chosen vaccine rollout strategy. *Subsequent allotments used to administer the first and second doses to 50% of a population; the other 50% of the population initially remain unvaccinated and are assumed to have no immunity against SARS-CoV-2 before start of vaccination. **Subsequent allotments used to administer second booster doses to 100% of a population. Efficacy of the first dose is assumed to be 85% from day 11 until day 27 after the administration of the first dose, assuming that the efficacy found during days 11 to 21 would continue to hold until day 27 (see Table 1). Efficacy after the second booster dose is assumed to be 95% from day 8 after the administration of the second dose onwards, based on data by Polack et al.1 As it is unclear how long the efficacy of 85% of the first dose will last, we assumed that the efficacy of the first dose would decrease to 70% from day 28 onwards until 7 days after administering the second booster dose in case of prolongations of the interval between first and second dose to 35 or 50 days. The intervals of 35 and 50 days were chosen arbitrarily for illustrative purposes. The time window starts at day 11 when the first dose of the vaccine is considered efficacious. Estimates only reflect the direct effects of the vaccine on vaccinated individuals but do not reflect the indirect effects on unvaccinated individuals through decreased transmission.
Figure 1 shows that administering Pfizer-BioNTech’s COVID-19 vaccine during the early stage of the vaccine rollout to as many individuals as possible could prevent more COVID-19 cases between 11 and 60 days after the start of the vaccine rollout compared to reserving half of the initial allotments as second booster doses. The longer the delay between the use of first allotments and availability of subsequent allotments, the larger the difference in the number of COVID-19 cases in favour of a strategy of administering Pfizer-BioNTech’s COVID-19 vaccine during the early stage of the vaccine rollout to as many individuals as possible.
Figure 2 shows the predicted cumulative incidence of COVID-19 cases according to rollout strategy and time of availability of subsequent allotments for the population included in Polack et al.’s Phase III trial1, as compared with the control group allocated to placebo. Curves divide early, and differences are always in favour of administering Pfizer-BioNTech’s COVID-19 vaccine during the early stage of the vaccine rollout to as many individuals as possible.
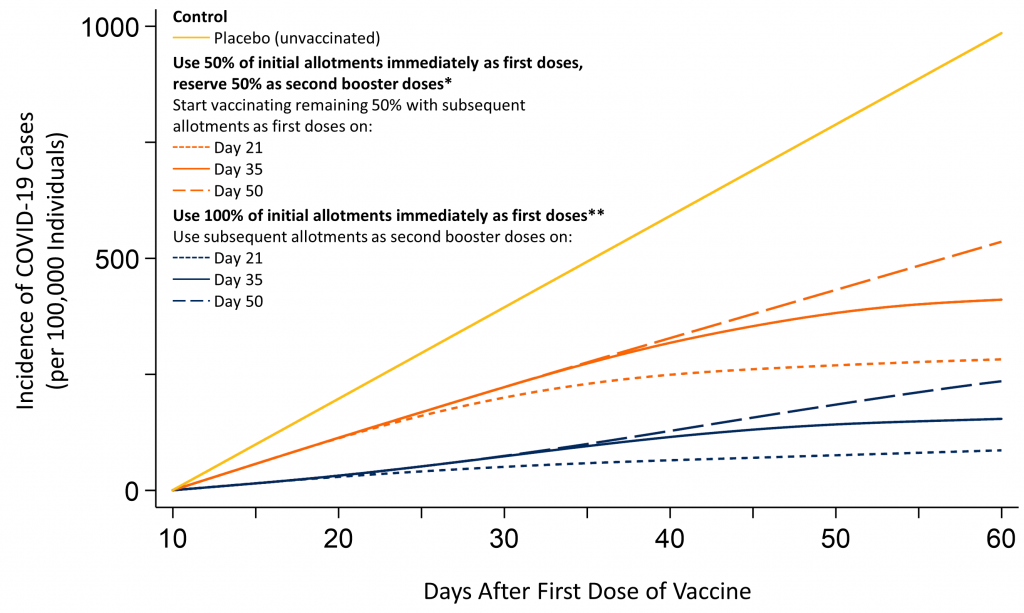
Predicted incidence of COVID-19 cases after vaccination with Pfizer-BioNTech’s COVID-19 vaccine, per 100,000 individuals included in Polack et al.’s Phase III trial,1 by vaccine rollout strategy and time of availability of subsequent allotments. *Subsequent allotments used to administer the first and second doses to 50% of a population. **Subsequent allotments used to administer second booster doses to 100% of a population. Efficacy of the first dose is assumed to be 85% from day 11 until day 27 after the administration of the first dose (Table 1). Efficacy after the second booster dose is assumed to be 95% from day 8 after the administration of the second dose onwards, based on data by Polack et al.1 As it is unclear how long the efficacy of 85% of the first dose will last, we assumed that the efficacy of the first dose would decrease to 70% from day 28 onwards until 7 days after administering the second booster dose in case of prolongations of the interval between first and second dose to 35 or 50 days. Estimates are for the population of participants included in the Phase III trial by Polack et al.1 and only reflect direct effects of the vaccine on vaccinated individuals but do not reflect indirect effects on unvaccinated individuals through decreased transmission.
A threshold analysis that assumes an interval of 50 days between the first and second doses indicates that the efficacy of the first dose would need to drop to less than 18% from day 28 onwards, for the initial strategy of reserving 50% of the initial allotments for use as second booster doses to become more effective than the alternate strategy of using 100% of the initial allotments immediately as first doses.
Interpretation
Administering Pfizer-BioNTech’s COVID-19 vaccine during the early stage of the vaccine rollout (January/February 2021) to as many individuals as possible could reduce the overall number of COVID-19 cases as compared to reserving half of the initial allotments as second booster doses in Ontario. An independent analysis using a decision analytical model came to the same conclusion (Tuite A, personal communication).6
On-label use of the vaccine with the administration of two doses is important, as the second dose significantly boosts the immune response and results in a substantial increase in neutralizing antibodies.3 It may also be important in assuring the overall durability of the immune response. However, using 100% of the initial allotments immediately to vaccinate as many individuals as possible does not preclude on-label use with two doses, with the second dose administered from a subsequent allotment as soon as it becomes available.
The occurrence of the novel SARS-CoV-2 variant7–9 is unlikely to change the conclusions of this Science Brief. Pfizer-BioNTech’s vaccine is likely to also be efficacious against the new variant.8 Assuming this similar vaccine efficacy, a higher risk of transmission associated with the new variant9 will result in an increased benefit of administering Pfizer-BioNTech’s COVID-19 vaccine during the early stage of the vaccine rollout to as many individuals as possible. This means that the reduction in COVID-19 cases afforded by a strategy of using 100% of initial allotments immediately will become larger with higher transmission. Conclusions will also remain unchanged even if there is a small to moderate decrease in efficacy of the vaccine against the variant.
The efficacy of Pfizer-BioNTech’s vaccine may be lower in the elderly.3 However, this is unlikely to negate the observed differences between the initial and the alternate vaccine rollout strategies.
Finally, the conclusions of this Science Brief also apply to Moderna’s COVID-19 vaccine.10 The estimated efficacy of the first dose of Moderna’s COVID-19 vaccine is 94% (95% CI 76 to 99%). This estimate is based on two versus 35 events that were reported in the vaccine and placebo groups of Moderna’s Phase III trial for the period starting 14 days after the first dose until the second booster dose was administered.11
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. Published online December 10, 2020. https://doi.org/10.1056/NEJMoa2034577
- National Advisory Committee of Immunization. Recommendations on the Use of COVID-19 Vaccines.; 2020. Accessed December 28, 2020. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html
- Walsh EE, Robert W. Frenck J, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. Published online October 14, 2020. https://doi.org/10.1056/NEJMoa2027906
- Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting December 10, 2020. FDA Briefing Document. Pfizer-BioNTech COVID-19 Vaccine.; 2020:53. https://www.fda.gov/media/144245/download
- Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH 1 T cell responses. Nature. 2020;586(7830):594-599. https://doi.org/10.1038/s41586-020-2814-7
- Grant K, Walsh M. New data favour administering COVID-19 vaccines as fast as possible, not reserving doses. The Globe and Mail. https://www.theglobeandmail.com/canada/article-new-data-favour-administering-covid-19-vaccines-as-fast-as-possible/. Published December 24, 2020. Accessed December 28, 2020.
- Government of Ontario. Ontario confirms first cases of COVID-19 UK variant in Ontario. Ontario Newsroom. Published December 26, 2020. Accessed December 28, 2020. https://news.ontario.ca/en/release/59831/ontario-confirms-first-cases-of-covid-19-uk-variant-in-ontario
- Rambaut A, Loman NJ, Pybus OG, et al. Preliminary Genomic Characterisation of an Emergent SARS-CoV-2 Lineage in the UK Defined by a Novel Set of Spike Mutations.; 2020. Accessed December 27, 2020. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563
- Davies NG, Barnard RC, Jarvis CI, et al. Estimated Transmissibility and Severity of Novel SARS-CoV-2 Variant of Concern 202012/01 in England. Centre for Mathematical Modelling of Infectious Diseases; London School of Hygiene & Tropical Medecine; 2020. Accessed December 27, 2020. https://cmmid.github.io/topics/covid19/uk-novel-variant.html
- Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting December 17, 2020. FDA Briefing Document. Moderna COVID-19 Vaccine.; 2020. Accessed December 17, 2020. https://www.fda.gov/media/144434/download
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. Published online December 30, 2020. https://doi.org/10.1056/NEJMoa2035389
Author Contributions: PJ and AMG conceived the Science Brief. PJ and YC wrote the first draft. All authors revised it critically for important intellectual content, and approved the final version.
Citation: Jüni P, Tuite AR, Bogoch II, et al. Rollout Strategy for the Pfizer-BioNTech COVID-19 Vaccine in Ontario. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2021;2(6). https://doi.org/10.47326/ocsat.2021.02.06.1.0
Author Affiliations: The affiliations of the members of the Ontario COVID-19 Science Advisory Table can be found at www.covid19-sciencetable.ca.
Declarations of Interest: The declarations of interest of the members of the Ontario COVID-19 Science Advisory Table can be found at www.covid19-sciencetable.ca.
Copyright: 2021 Ontario COVID-19 Science Advisory Table. This is an open access document distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original work is properly cited.
