The Incidence, Severity, and Management of COVID-19 in Critically Ill Pregnant Individuals
Authors:Laveena Munshi, Julie K. Wright, Jonathan Zipursky, Sarah Jorgensen, Tali Bogler, Katherine J. Miller, Maha Al Mandhari, Kali Barrett, Shital Gandhi, Serena Gundy, Andrew Healey, Peter Jüni, Gabrielle M. Katz, Andrew M. Morris, Menaka Pai, Anna Perkhun, Joel G. Ray, Prakeshkumar Shah, Stephen E. Lapinsky*, Wendy L. Whittle* on behalf of the Ontario COVID-19 Science Advisory Table. *SL and WW are senior co-authors for this Science Brief.
Key Message
The rate of SARS-CoV-2 infection in pregnancy does not appear to be higher than in the general population; however, compared to their non-pregnant counterparts, pregnant individuals have higher morbidity and mortality, with a higher risk of intensive care unit (ICU) admission, mechanical ventilation, and need for extracorporeal membrane oxygenation (ECMO). They also have a higher frequency of pre-eclampsia, Cesarean delivery, and a higher rate of preterm birth.
Care of the critically ill pregnant patient with COVID-19 requires a multidisciplinary team that includes obstetrics, neonatology, anesthesia, infectious diseases, medicine, and critical care.
Potentially life-saving evidence-based therapies such as corticosteroids and tocilizumab should not be withheld from pregnant individuals with severe COVID-19.
Vaccines against SARS-CoV-2 are safe to use among pregnant individuals and vaccination is highly recommended in this population.
Summary
Background
Since the emergence of SARS-CoV-2, there has been concern for the vulnerability of the pregnant population to COVID-19. Historically, pregnant individuals have been at higher risk for adverse medical and obstetrical outcomes during viral respiratory outbreaks. Immunologic, respiratory, and anatomic changes that occur during pregnancy may explain the greater susceptibility to more severe disease.
Questions
What is the incidence of SARS-CoV-2 infection in pregnant individuals compared to non-pregnant reproductive-aged peers?
Among pregnant individuals with SARS-CoV-2 infection, what is the rate of hospital admission, ICU admission, and mechanical ventilation for COVID-19 respiratory disease?
What are the outcomes (maternal, obstetrical, and neonatal) across hospitalized and critically ill pregnant patients with COVID-19 acute respiratory failure?
What are the unique management considerations for critically ill pregnant patients with COVID-19?
Findings
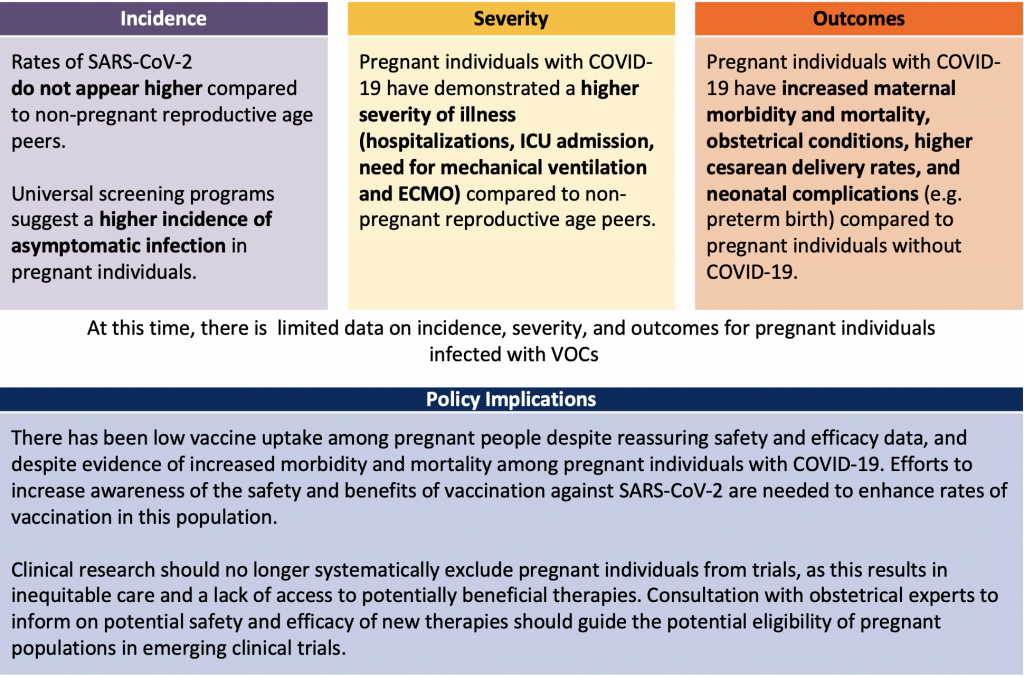
Available data suggest that the incidence of symptomatic SARS-CoV-2 infection is not higher in the pregnant population compared to the general population; however, incidence is difficult to delineate from existing international data. There appears to be a higher incidence of asymptomatic infection in the pregnant population.
Seven to fifteen percent of pregnant individuals with COVID-19 will experience moderate to severe disease requiring hospitalization. Canadian and international data suggest that pregnant individuals with SARS-CoV-2 infection have a higher risk of ICU admission, mechanical ventilation, and need for ECMO. More data surrounding VOCs in this population is needed to understand whether they pose a higher risk of illness severity.
Maternal morbidity and mortality is higher in pregnant individuals with COVID-19 compared to those without the virus. Poor obstetrical and neonatal outcomes have also been reported, though very few Canadian infants tested positive for SARS-CoV-2 after birth from COVID-19 positive pregnant individuals. This suggests that the rate of transmission to the fetus during delivery is low.
There is limited high-quality data on the management of severe COVID-19 acute respiratory failure in pregnant individuals. Pregnant patients with severe COVID-19 should ideally be cared for by multidisciplinary teams familiar with the management of pregnant patients with respiratory failure. Principles of management that apply to non-pregnant patients with severe COVID-19 should be applied to pregnant patients, including the use of evidence-based medications such as corticosteroids, tocilizumab, and interventions such as prone positioning for mechanically ventilated patients and extracorporeal membrane oxygenation (ECMO), when indicated. In the setting of severe acute respiratory distress syndrome (ARDS), optimal timing of delivery should be discussed with the obstetrical care provider. Decision-making for delivery in critically ill obstetrical patients with COVID-19 acute respiratory failure should involve obstetrical care, maternal fetal medicine, critical care, anaesthesia, and neonatal providers and take into consideration the gestational age, health of the fetus, and severity of illness of the patient.
While pregnant individuals were excluded from the clinical trials of mRNA and viral vector COVID-19 vaccines, several published analyses of large cohorts have demonstrated effectiveness of vaccines in reducing the likelihood of acquiring SARS-CoV-2 and there have been no concerning safety signals reported among pregnant vaccine recipients.
Interpretation
Whereas the overall incidence of SARS-CoV-2 respiratory infection may be low, pregnant individuals infected with SARS-CoV-2 are at increased risk of developing severe COVID-19 and adverse pregnancy and neonatal outcomes. At the time of writing this Science Brief, there is limited evidence on the impact of SARS-CoV-2 VOCs on the incidence, severity, outcomes, and management of pregnant individuals compared to the general population. Despite the safety and efficacy of COVID-19 mRNA vaccines and the heightened risk for severe disease in the pregnant population, vaccine uptake in pregnant individuals is lower than other higher-risk populations. Vaccine hesitancy is, in part, driven by the failure to include pregnant individuals in initial vaccine trials. Tailored messaging to increase vaccine confidence is needed — particularly considering more virulent VOCs. Finally, future clinical trials during respiratory disease epidemics and pandemics should include pregnant individuals. In collaboration with experts in obstetrical care, methods for rapidly evaluating the safety and efficacy of novel therapies should be pursued to determine eligibility of pregnant individuals for therapeutic trials. It is essential to accumulate high-quality data for this particularly vulnerable group given their immunologic and physiologic susceptibility to developing severe respiratory failure.
Full text
Background
Since the emergence of SARS-CoV-2, there has been concern for the unique vulnerability of the pregnant population to COVID-19. Immunologic and respiratory changes that occur during pregnancy may explain the greater susceptibility to severe disease, alongside the anatomic and physiologic changes that occur with advancing gestation.1–4 Increased oxygen consumption and minute ventilation, decreased functional residual capacity, airway edema, and transient immunosuppression with altered T-cell mediated immunity render pregnant individuals susceptible to severe infection. Historically, the pregnant population has experienced increased illness severity and adverse outcomes during viral respiratory infection outbreaks, such as the H1N1 influenza pandemic of 1919, the H2N2 influenza pandemic in 1959, the 2009 H1N1 influenza pandemic, and MERS-CoV.1–4 The objectives of this brief are to evaluate the incidence, severity, and outcomes of COVID-19 in pregnant individuals and describe unique management considerations for those with moderate to severe COVID-19.
Questions
What is the incidence of SARS-CoV-2 infection in pregnant individuals compared to non-pregnant reproductive-aged peers?
Among pregnant individuals with SARS-CoV-2 infection, what is the rate of hospital admission, ICU admission, and mechanical ventilation for COVID-19 respiratory disease?
What are the outcomes (maternal, obstetrical, and neonatal) across hospitalized and critically ill pregnant patients with COVID-19 acute respiratory failure?
What are the unique management considerations for critically ill pregnant patients with COVID-19?
Findings
Characterizing Incidence
It is difficult to characterize the incidence of SARS-CoV-2 infection in the pregnant population because of the variability in testing thresholds, data collection methods, and community prevalence of COVID-19 from international reports.5–10Testing strategies varied by jurisdiction and ranged from universal testing of all pregnant individuals to screening/symptom-based testing at the time of delivery.
The PregCOVID living systematic review was established early in the pandemic to evaluate clinical manifestations, risk factors, and outcomes of pregnant individuals with COVID-19.7 In their latest publication with data through October 2020, which included 73 studies and 67,271 pregnant individuals, 10% of pregnant or recently pregnant patients admitted to hospital for any reason had suspected or confirmed COVID-19.7
The Canadian Surveillance of COVID-19 in Pregnancy team (CANCOVID) was established at the beginning of the pandemic and regularly reports on the epidemiology and maternal and infant outcomes of patients with COVID-19. The reports include data from 5 Canadian provinces (Ontario, Manitoba, British Columbia, Quebec, and Alberta).6 According to the most recent report (released on June 3, 2021) reflecting the time period from March 2020 to March 31, 2021, 4,805 cases of COVID-19 in pregnancy had been collected. The rate of SARS-CoV-2 infection in pregnancy varied by province, ranging between 0.9 to 2.8%. The rate of infection in non-pregnant women of similar age during the same time period was 2.6 to 6.7%. In Ontario specifically, 1,358 pregnant individuals had tested positive for SARS-CoV-2, representing 0.89% of the pregnant population. This incidence is lower than that of non-pregnant reproductive aged peers (2.99%); however, the reporting period was largely prior to the emergence of the more transmissible Alpha (B.1.1.7) or Delta (B.1.617.2) VOCs in Ontario.
Furthermore, according to reports from universal screening programs at the time of delivery in the UK, Belgium, and New York, pregnant individuals had a higher incidence of asymptomatic infection than their non-pregnant peers, despite similar incidence of COVID-19.7–10 It is possible that the incidence could be underestimated in Canada given the absence of universal screening and/or testing.
Maternal Demographics
CANCOVID data indicates that the second and third trimesters were the most common time in pregnancy for SARS-CoV-2 infection.6 Gestational age was less than 14 weeks in 20.9% of cases, 14 to 27 weeks in 40.1% of cases, 28 to 38 weeks in 31.6% of cases, and >38 weeks in 7.5% of cases. Awareness of pregnancy could have underestimated the incidence in the first trimester. Obesity was the most common underlying maternal condition (12.9%), followed by diabetes (11.2%). The majority of Canadian pregnant individuals presenting with COVID-19 were 30-34 years of age. Notably, maternal age greater than 35 was identified as a risk factor for infection. This could be due to an increased incidence of maternal co-morbidities in this age group given that it is typically a population identified as higher obstetrical risk. Overall, risk factors for the acquisition of SARS-CoV-2 appeared to mirror those in the general population including being a member of a racialized community, low-income status, or living in neighborhoods with increased population densities.11 A large international study also demonstrated that the presence of diabetes, hypertension and pulmonary disease were risk factors for COVID-19 infection during pregnancy.12
In summary, the incidence of COVID-19 does not appear to be higher in pregnancy. However, there may be an increase in asymptomatic infection in pregnant individuals. The second and third trimesters are the most common time in pregnancy to acquire SARS-CoV-2 infection. There is limited information regarding the profile of the pregnant individual diagnosed with COVID-19 due to VOCs. The increased transmissibility of VOCs may impact the identified risk factors for infection, increasing the vulnerability of the pregnant population.
Characterizing Severity
Canadian and international data indicate that the majority (85 to 90%) of pregnant individuals infected with SARS-Cov-2 were asymptomatic or had mild disease.6-10 However, 7 to 15% of pregnant individuals with COVID-19 experienced moderate to severe disease requiring hospitalization,7 admission to the ICU, mechanical ventilation, or consideration for ECMO. Compared with non-pregnant reproductive aged peers diagnosed with COVID-19, the relative rates of hospitalization and admission to ICU were significantly increased (RR 4.26 95% CI 3.45-5.10 – hospitalization; RR 11.39 95% CI 7.90-15.21 0 ICU admission).6 Of pregnant individuals with data available in CANCOVID, 7.1% of pregnant individuals with COVID-19 were admitted to hospital and 2.5% were admitted to the ICU (compared to 1.5% and 0.25%, respectively, of non-pregnant reproductive aged peers). The increased relative risk may reflect the exacerbation of the disease by the physiology of pregnancy and/or a lower threshold for admission.
Similar increases in ICU admission of pregnant patients with COVID-19 were noted in reports from the US, UK, and Italy.13,14 The INTERCOVID multinational cohort study of 43 institutions across 18 countries reported increased risk of ICU admission and referral to higher levels of care.13 International rates for ICU admission were 5 to 7% in pregnant hospitalized patients with COVID-19.
While the decision to admit pregnant patients with COVID-19 to hospital or ICU may reflect a lower threshold to advance care for this population, the corresponding need for mechanical ventilation reported in many studies (2 to 6% of pregnant individuals in the INTERCOVID study) is indicative of clinically severe disease.13 According to the PregCOVID living systematic review of 192 cohort studies of pregnancy outcomes across hospitalized patients with COVID-19 during the first wave, the odds ratio (OR) for ICU admission was 2.13 (95% CI 1.53 to 2.95), need for invasive mechanical ventilation was 2.59 (95% CI 2.28 to 2.94), and need for ECMO was 2.02 (95% CI 1.22 to 3.34) compared to non-pregnant females of similar age.7 Pregnant individuals with COVID-19 were hospitalized 3.73 days longer than pregnant individuals without COVID.7
The Impact of Variants of Concern on Severity of Illness
Over a series of reports from CANCOVID, the relative risk of hospitalization and ICU admission increased.6 However, the most recent report partially incorporates the timeframe during which the Alpha variant was circulating, suggesting the increased relative risk of moderate to severe disease in the pregnant population may be driven by the VOCs.6 In a preprint describing emerging VOCs in Ontario from February to June 2021, 566 out of 200,000 patients were pregnant, with 74% (419) having a VOC.14 After adjusting for relevant confounders, pregnant individuals had an OR of 6.27 (95% CI 4.47 to 8.60) for hospitalization and OR 6.46 (95% CI 3.43 to 12.21 for ICU admission compared to the general population.22
According to the March 2021 UK Obstetrical Surveillance System/ISARIC Coronavirus Clinical Characterization Consortium, COVID-19 Clinical Information Network (UKOSS/ISARIC/CO-CIN) report from the UK, there was a greater proportion of recently pregnant (within 6 weeks) or pregnant individuals with confirmed COVID-19 admitted from September 2020 to March 2021 (wave 2 — 293 pregnant out of 2157 females aged 16 to 49; 14%) compared to March 2020 to August 2020 (wave 1 — 70 pregnant out of 790 females aged 16 to 49; 9%).15 There was a higher proportion of pregnant patients requiring invasive mechanical ventilation in the first 24 hours of hospital admission from September 2020 to March 2021 (wave 2 — 93 out of 686 females aged 16 to 49; 13.5%) compared to March to August 2020 (wave 1 — 31 out of 376 females aged 16 to 49; 8.2%). The Alpha variant was first detected in the UK in November 2020 from a sample taken in September 2020; therefore, this higher incidence noted in the pregnant population suggests a higher severity of illness with the Alpha variant. There was no data available at the time of the evidence synthesis on the severity of illness with the Delta variant. The Royal Brompton Hospital, one of five ECMO centers in the UK, reported increased referrals for ECMO for women aged 16 to 49 with COVID-19 during the second wave, with a large proportion being pregnant or recently pregnant (Wave 1: 12%; Wave 2: 28%, p=0.047).16 These reports convey an increased utilization of critical care resources for pregnant individuals infected with VOCs. However, it is not known whether these reflect changes in demographics or whether the emerging VOCs pose an augmented risk unique to pregnant individuals compared to the general population.
Characterizing Outcomes
Evidence to date suggests that pregnant patients with COVID-19 acute respiratory failure are at increased risk of maternal morbidity and mortality, and poor neonatal outcomes compared to pregnant patients without COVID-19.7 The impact of COVID-19 in pregnancy on maternal, obstetrical, and neonatal outcomes has been described in a variety of populations both nationally and internationally.
Maternal Outcomes
The INTERCOVID cohort study reported an increased risk of a composite of maternal morbidity or mortality (risk ratio(RR) 1.54) in pregnant individuals with COVID-19 compared with pregnant individuals without COVID-19, and a 22-fold increased risk of maternal death.13 The odds ratio (OR) for all-cause mortality was 2.85 (95% CI 1.08 to 7.52) compared to pregnant individuals without COVID-19 according to the PregCOVID systematic review (8 studies across 4,820 pregnant individuals; data available until October 2020).7
However, whether pregnant individuals with COVID-19 are at higher risk of death compared to non-pregnant women of similar age with COVID-19 remains unknown. Based on data in the PregCOVID living systematic review (59 studies across 41,664 pregnant individuals with COVID-19), the overall mortality in pregnant individuals remains low (0.02%, 95% CI 0.00 to 0.42) .7 In this report, there was no increase in all-cause mortality when compared to non-pregnant women of reproductive age with COVID-19 (OR 0.96; 95% CI 0.79 to 1.02). However, these data do not reflect outcomes of patients infected with VOCs.
Risk Factors for Disease Severity and Mortality
Several important risk factors for disease severity and need for critical care among pregnant individuals with COVID-19 have been identified and mirror the general population. High body mass index (greater than 30) was associated with increased risk of severe COVID-19 (OR 2.37), admission to ICU (OR 2.71), need for invasive ventilation (OR 6.61), and death (OR 2.27).7 Non-white ethnicity was associated with increased risk of admission to ICU (OR 1.66), invasive ventilation (OR 2.23), and death (OR 1.61).7 Increasing maternal age (>35 years in 6 out of 7 studies) was associated with risk of developing severe COVID-19 (OR 1.83) and admission to ICU (OR 2.11).7 One possible interpretation of the existing data is that the existence of comorbidities in pregnant individuals may explain the higher severity of illness compared to non-pregnant individuals.
The World Association of Perinatal Medicine Working Group on COVID-19 evaluated high versus low-risk pregnancies complicated by severe COVID-19 across 76 centers and 25 countries (published February 20, 2021). Higher risk pregnancies, defined by the presence of pre-existing chronic medical conditions (e.g., chronic hypertension/diabetes) or obstetrical disorders (e.g., pre-eclampsia, gestation hypertension, or gestation diabetes), were at higher risk for ICU admission, use of mechanical ventilation, or adverse maternal morbidity/mortality compared to low-risk pregnancies.17
Obstetrical and Neonatal Outcomes
An increased rate of preterm birth, low birth weight, pre-eclampsia, and Cesarean delivery has been described with COVID-19.6,8,12,18 Among pregnant individuals with COVID-19 who delivered in Canada, 97% of births were live births, 63% were vaginal deliveries, and 37% were Cesarean sections driven by obstetrical, fetal, or medical indications.6 The rate of preterm birth was 12.3% in pregnant individuals with COVID-19, which is higher than the pre-pandemic period (8.3% baseline rate).6 Thirty-four percent of patients with COVID in pregnancy delivered preterm with a mean gestational age of 34-37 weeks, a gestational age typically associated with limited neonatal morbidity and/or mortality. The majority of infants were in the normal range for birth weight and approximately 15% were admitted to the neonatal ICU.
International reports show similar obstetrical and neonatal outcomes for pregnancies complicated by COVID-19 as seen in Canada;4,8,13,18 however, the majority reflect infection when the wild-type strain was predominant. According to the INTERCOVID cohort study, there was a higher incidence of pre-eclampsia/eclampsia in pregnant individuals with COVID-19 (RR 1.76; 95% CI 1.27 to 2.43) compared to women without COVID-19.13,19 This may be due to the overlap between COVID and pre-eclampsia risk factors. There was a greater incidence of Cesarean delivery (RR 1.28 95% CI 1.16 to 1.40) which was predominantly deemed to be medically indicated (RR 1.97, 95% CI 1.56 to 2.51) and a greater risk of preterm birth (less than 37 weeks gestation) (RR 1.59, 95% CI 1.56 to 2.51). There have been inconsistent reports on the impact of COVID-19 in pregnancy and the rate of stillbirth and these rates may reflect local variation and pregnancy risk. Reassuringly, there have not been any reports of teratogenicity, first trimester spontaneous abortion, or significant impact on fetal growth attributed to SARS-CoV-2.
Despite potential for in-utero and immediate-newborn impact given the presence of the ACE2 receptor in placenta and fetal tissues, there are limited reports of COVID-19 infection in the newborn population. The modest increased rate of neonatal ICU admission can be attributed to the increased rate of preterm birth and not a direct impact of COVID-19 on the newborn. The majority of babies born at the time of active maternal COVID-19 infection test negative (less than 90%); of the babies who tested positive, approximately 2 out of 3 of cases were attributed to horizontal transmission after delivery.6,20 In Canada, 237 infants were tested for SARS-CoV-2 infection after birth with fewer than 6 having a positive result.6,21 There are reported cases of possible Multisystem Inflammatory Syndrome of neonates.22,23
Risk Factors for Poor Obstetrical and Neonatal Outcomes
The majority of studies on obstetrical and neonatal outcomes of SARS-CoV-2 infection in pregnancy do not stratify by COVID-19 disease severity. However, there is some preliminary evidence demonstrating that, compared to mild COVID-19, severe COVID-19 was more strongly associated with the development of pre-eclampsia, preterm birth, Cesarean delivery, low birth weight, and neonatal ICU admission.24 Similarly, data from the UKOSS/ISARIC/CO-CIN report suggest that pregnant patients with COVID-19 acute respiratory failure may be at increased risk of morbidity and mortality, adverse obstetrical events, and neonatal outcomes compared to pregnant patients without COVID-19.15
Management Considerations Unique to the Obstetrical Population with Severe COVID-19
Organizational Considerations
Pregnant patients with severe acute respiratory failure due to COVID-19 should be admitted to a centre with pregnancy and COVID-19 expertise appropriate for the gestational age of the patient including critical care, obstetrical care, neonatal ICU, obstetrical anesthesia, obstetrical medicine, and high-risk obstetrical care providers. This may require transfer of the patient to an institution capable of providing this multidisciplinary care. If transfer is not feasible and/or all types of expertise are not available at one site, consideration should be given to the creation of virtual teams that include off-site high-risk obstetrical medicine experts, maternal fetal medicine experts and/or critical care physicians with expertise in the management of pregnant patients with respiratory failure, as well as neonatal transport capability where needed.
Acute Respiratory Failure Management
In the setting of acute respiratory failure requiring invasive mechanical ventilation, principles of care should mirror that of the non-COVID-19 pregnant patient.
Traditionally, oxygen saturation in the pregnant individual is maintained at or above 94% based on animal studies reflecting changes in fetal behaviours (breathing and movement), heart rate, and middle cerebral artery blood flow in response to incremental decreases in saturations lower than 94%.25 However, generalizability of these findings may be limited given differences in placental anatomy and physiology. It is well understood that fetal hemoglobin has a higher affinity for oxygen than adult hemoglobin, a factor which promotes fetal oxygenation; a passive gradient of diffusion from fetal to maternal circulation allows for fetal excretion of carbon dioxide. However, fetal oxygenation is not dependent solely on maternal oxygen saturation; oxygen delivery to the fetus is dependent on maternal oxygen content (hemoglobin and oxygen saturation) and placental blood flow determined by maternal cardiac output. As such, fetal surveillance parameters (e.g., heart rate by non-stress test, biophysical profile) will be influenced by factors other than maternal oxygen saturation including sedation/paralysis and/or the symptoms of disease (e.g., maternal fever, tachycardia) in the setting of mechanical ventilation and as such become difficult to interpret. Use of fetal Doppler studies (umbilical artery and fetal ductus venosus Doppler pattern) may be useful to determine the impact of maternal oxygenation on fetal status.
There is limited data specific to the pregnant population with COVID-19 surrounding timing of intubation, use of non-invasive oxygen strategies, mechanical ventilation, sedation management, and use of adjuvant strategies (e.g., prone positioning, extracorporeal life support, inhaled nitric oxide, etc.). Intubation of a pregnant patient is considered higher risk given the anatomic and physiologic changes during pregnancy; therefore, if available in a timely manner, intubation should be conducted by an expert airway provider. Pregnant patients were excluded from many randomized controlled trials of COVID-19 therapies and interventions for acute respiratory failure. There is no role for routine computerized tomography (CT) scans of the chest for any patient with COVID-19 and should be reserved for when clinically indicated (e.g., ruling out pulmonary embolism).
Prone Positioning in Mechanically Ventilated Patients
Prone positioning in the setting of severe ARDS is associated with decreased mortality and has been adopted in the management of COVID-19 ARDS.26–28 Prone positioning is low cost, and, for the general population, associated with low numbers of adverse events.27 There has been historic a reluctance to adopt prone positioning for pregnant patients given perceived challenges or concerns about the impact on the fetus. Pregnant patients were excluded from many of the early randomized trials on prone positioning. At the beginning of the pandemic, some guidelines stated that prone positioning was contraindicated in the second and third trimesters of pregnancy, citing the lack of guidance available in pregnancy.29
Despite these early recommendations against prone positioning in this population, it may help to relieve both diaphragmatic and aortocaval compression on the lungs, which is beneficial in ARDS.30 Case reports have described the safety of prone positioning in pregnant patients with ARDS, demonstrating feasibility and improvements in oxygenation.31–33 However, most case reports describe prone positioning at or less than 26 weeks gestation. The physiological considerations of the large gravid uterus in late pregnancy and interference with fetal heart monitoring should be considered. Towards the end of the third trimester there may be a greater tendency to deliver if the pregnancy is complicated by severe ARDS as opposed to considering maneuvers like prone positioning. In the absence of alternative evidence, indications for prone positioning remain the same as in non-pregnant patients. Clinical guidelines and algorithms for the safe proning of pregnant individuals have been published and are available online.30 Discussions about timing of delivery should be made with the obstetrical team if prone positioning is being considered to help improve maternal oxygenation. Prone positioning has been employed successfully in post-partum patients with monitoring of the incision in the setting of Cesarean delivery.
Mode and Timing of Delivery
The diagnosis of COVID-19 in pregnancy is not an indication for delivery, regardless of gestational age. There is no evidence informing optimal timing of delivery for the pregnant patient with moderate to severe acute respiratory failure due to COVID-19 compared to pregnant patients with moderate to severe acute respiratory failure due to other respiratory illnesses. Timing of delivery should be individualized and take into consideration the clinical status of the patient, the impact of pregnancy on their state of critical illness, maternal medical and/or obstetrical comorbidities (e.g., pre-eclampsia), gestational age, and fetal condition.
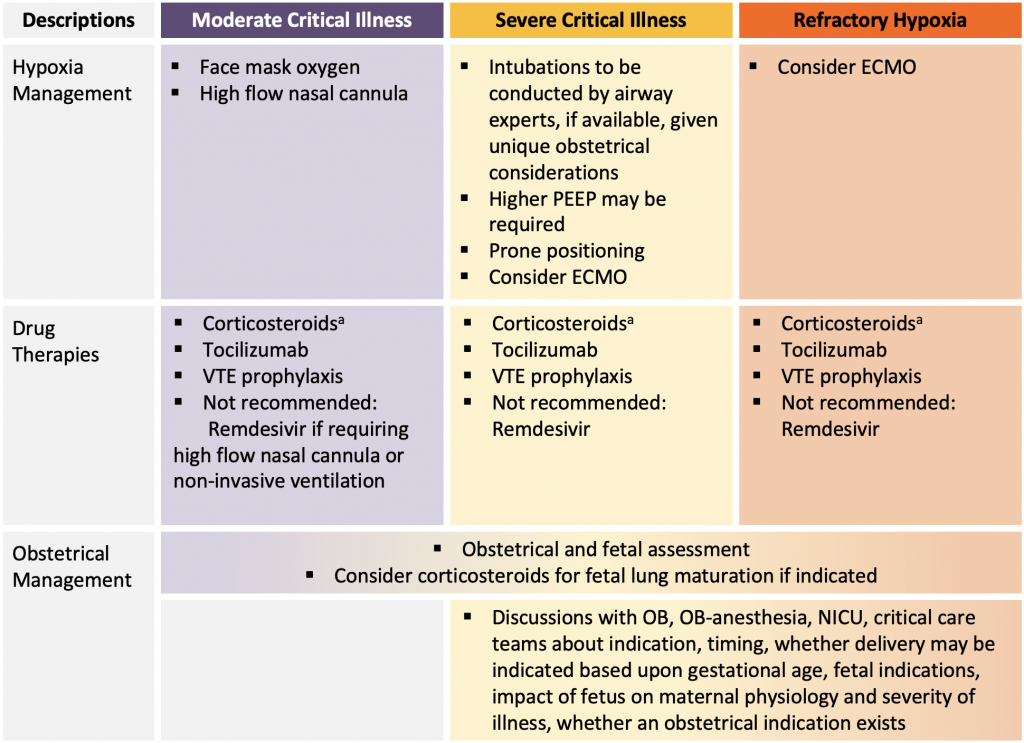
Figure presenting a summary of available evidence for COVID-19 management in pregnancy. aMany institutions adopted the following approach to corticosteroids: If currently less than 22 or greater than 36 weeks gestation: methylprednisolone 32 mg IV x 1 dose, followed by methylprednisolone 32 mg IV (or prednisone 40 mg orally) daily for days 2-10. If currently 22 to 36 weeks gestation: dexamethasone 12 mg IV daily (or dexamethasone 6mg IV twice a day) for 2 days for fetal lung maturation followed by methylprednisolone 32 mg IV daily (or prednisone 40 mg orally daily) for days 3-10 (55). If post-partum (with or without breastfeeding) dexamethasone 6 mg orally or IV daily for 10 days or until hospital discharge. PEEP, positive end-expiratory pressure. ECMO, extracorporeal membrane oxygenation. OB, obstetrician or obstetrics. NICU, neonatal intensive care unit.
Although delivery may improve maternal respiratory parameters in some patients, this is not a universal occurrence, and there is potential for maternal deterioration related to the physiological stress and fluid shifts of anesthesia and delivery. In late pregnancy, there may be unique organizational considerations influencing timing of delivery if ECMO is being considered and the ECMO centre does not provide Obstetrical and Neonatal services. Management considerations are outlined in Figure 2.
Medications
According to the US National Institute of Health, potentially effective treatments for COVID-19 should not be withheld from pregnant women with severe acute respiratory failure because of theoretical concerns related to the safety of therapeutic agents.
Corticosteroids
Corticosteroids have been associated with decreased mortality in hospitalized patients requiring oxygen with COVID-19.34,35 Pregnancy and breastfeeding were not exclusions in the RECOVERY trial, the landmark randomized control trial demonstrating the effectiveness of corticosteroids in SARS-CoV-2 infection. However, only 4 pregnant patients were enrolled in the corticosteroid platform (with only 1 in the corticosteroid arm). The reservation for enrollment could possibly be related to the uncertain impact of corticosteroids on the fetus. Indeed, some studies have documented associations with intrauterine growth restriction, preterm birth, gestational diabetes, and pre-eclampsia.36 However, most studies do not adequately account for treatment indication or illness severity and these results need to be interpreted with caution.
The association between corticosteroid use and oral clefts is well studied, and the evidence does not show an increase the incidence of oral clefts associated with maternal corticosteroid use above that found the general population (1.7 per 1,000 births).36–40 No adverse events have been documented in breastfed infants of mothers taking systemic corticosteroids. Amounts of corticosteroids in breastmilk are low and are considered safe if used for short durations while breastfeeding. There is limited data on dexamethasone during breastfeeding. High-dose corticosteroids may temporarily decrease milk supply.
In the RECOVERY TRIAL, for pregnant patients, prednisolone (40 mg by mouth daily) or hydrocortisone (80 mg IV twice daily) were suggested as alternatives to dexamethasone.35 Prednisolone and hydrocortisone are extensively metabolized by placental 11 beta hydroxysteroid dehydrogenase type 2 to an inactive metabolite to protect the fetus from corticosteroid exposure.41 Other short acting corticosteroids such as methylprednisolone and prednisone share this property of reduced placental transfer.41 By contrast, fluorinated corticosteroids (dexamethasone and betamethasone) have the highest rate of placental transfer with minimal mineralocorticoid effects and are recommended in the antenatal period to induce fetal lung maturity in women at high risk for preterm birth. Historically, methylprednisolone has been the preferred corticosteroid for the treatment of ARDS in both non-pregnant and pregnant adults due to better lung penetration.42–45
Institutional practices vary on the type of corticosteroids administered during pregnancy in the setting of acute respiratory failure due to COVID-19 for pregnant patients. Some institutions recommend the following: If currently less than 22 or greater than 36 weeks gestation: methylprednisolone 32 mg IV x 1 dose, followed by methylprednisolone 32 mg IV (or prednisone 40 mg orally) daily for days 2-10. Given the increased risk of preterm birth associated with any critical maternal illness, if currently 22-36 weeks gestation: dexamethasone 12 mg IV daily (or dexamethasone 6mg IV or IM twice a day)46–48 for 2 days for fetal lung maturation followed by methylprednisolone 32 mg IV daily (or prednisone 40 mg orally daily) for days 3-10.48 If post-partum (with or without breastfeeding) most recommend dexamethasone 6 mg orally or IV daily for 10 days or until hospital discharge. Blood glucose, particularly in the setting of gestational diabetes, should be monitored closely.
Remdesivir
Remdesivir is an antiviral agent that may shorten time to recovery in hospitalized moderately ill patients with COVID-19 requiring oxygen therapy but not those who were critically ill.49 Pregnant and breastfeeding patients were excluded from all 5 randomized trials evaluating remdesivir for COVID-19; as such, there is no direct evidence of efficacy in this population beyond observational studies.50–54
Remdesivir has been evaluated in reproductive toxicology studies using animal models and no adverse findings were observed with exposures up to 4 times higher than those achieved in humans with recommended dosing.55 All published reports of clinical use of remdesivir in pregnancy have included patients in the second or third trimester of pregnancy, or, in some cases, the timing of exposure was not described. As such, the risks of exposure at conception (or in early pregnancy) on major malformations or spontaneous abortions are unknown.
In the largest report of remdesivir in pregnancy (n=156), using data from Gilead’s global safety database, there were 33 live births, 13 adverse pregnancy outcomes (7 spontaneous abortions, 2 induced abortions, and 4 still births), and 110 unknown outcomes.56 Of the 13 cases with adverse pregnancy outcomes, 9 were reported in patients treated with remdesivir for Ebola virus infection and 4 received remdesivir for COVID-19. All patients that experienced adverse pregnancy outcomes (n=4) were critically ill and required invasive mechanical ventilation within 24 hours of starting remdesivir. Five infants with congenital abnormalities were identified, but remdesivir exposure occurred after the first trimester in all cases. Additional cohort studies, case series, and reports of remdesivir in pregnancy have not raised new safety concerns but lack appropriate comparator groups to properly assess relevant maternal and neonatal outcomes.32,57–65 Increases in transaminases have been described in both pregnant and non-pregnant individuals treated with remdesivir.57,61,65,66 Liver enzyme abnormalities might be related to remdesivir, COVID-19, other pregnancy-related (e.g., preeclampsia), or unrelated causes.
There are no data describing remdesivir pharmacokinetics in pregnancy. However, simulation studies suggest pregnancy-related increases in glomerular filtration rate and renal tubular secretion may increase elimination of active metabolites.67 Additionally, changes in plasma protein concentrations due to drug displacement from protein binding sites and volume expansion may alter unbound concentrations of remdesivir and its metabolites.67 For these reasons, pregnant people may require higher doses than non-pregnant populations. Moreover, remdesivir is rapidly hydrolyzed to a nucleoside monophosphate analogue and requires several additional steps of metabolism to generate the active intracellular nucleotide triphosphate analogue.66 This suggests that remdesivir itself is unlikely to transfer across the placenta in clinically important amounts, but characteristics of major circulating metabolites (e.g., long half-life, low molecular weight, high unbound fraction) suggest that they may.
There is no data on the use of remdesivir in breastfeeding individuals. Remdesivir has poor oral bioavailability; therefore, infants are unlikely to absorb clinically important amounts from breastmilk.68 Furthermore, no adverse effects were documented in a small number of infants who were treated with remdesivir for Ebola virus infection or COVID-19.69,70
Tocilizumab
Tocilizumab has been shown to reduce the composite endpoint of mechanical ventilation and mortality in critically ill patients with COVID-19 acute respiratory failure and moderately ill patients with disease progression.71–80 Of the 9 randomized controlled trials that evaluated tocilizumab for COVID-19, only the RECOVERY trial enrolled pregnant individuals (n=10, 0.2% of subjects).72,78 Specific maternal and neonatal outcomes were not reported for this sub-group.
Monoclonal antibodies are actively transported across the placenta, facilitated by the binding of the Fc portion to receptors on the placenta.81 During the first trimester, Fc receptors are barely expressed and antibody levels in the fetus are low. Fetal tocilizumab exposure is therefore likely negligible during the critical period of organogenesis. Antibody transport increases during pregnancy and is highest in the third trimester.81 Pre-clinical reproductive toxicology studies in monkeys show no evidence of teratogenicity with the administration of tocilizumab in the first trimester.82 However, there were dose-related increases in the incidence of abortion or embryo-fetal death at higher exposures. Additionally, in mice experiments, offspring of dams treated with tocilizumab showed signs of mild immunosuppression.82
Of the pregnant individuals treated with tocilizumab and followed prospectively in the Roche Global Safety database (n=180) there was no increase in congenital malformations compared to the baseline rates in the general population (tocilizumab: 4.5%; population: 3.0 to 6.6%).83 These data are consistent with the most recent EULAR report (n=218) which detected 3.9% congenital malformations.84 Spontaneous abortions were higher than in the general population (15-20%) in both the Global Database (21.7%) and the EULAR report (21.6%). However, concomitant methotrexate, which was prescribed to approximately 20% of patients and is a known teratogen, may have impacted these outcomes.83–85
There have been 3 reports of tocilizumab use in pregnant people with COVID-19.62,86,87 Tocilizumab administration often occurred in the third trimester, many patients were critically ill, and corticosteroids were given to a minority of patients. In the largest series from Spain (n=12), all pregnancies resulted in live births, but most had limited neonatal follow-up.86 There was one case of maternal cytomegalovirus (CMV) reactivation and congenital CMV infection.86
The pharmacokinetics of tocilizumab have not been characterized in pregnancy. However, based on what is known about monoclonal antibody pharmacokinetics and changes associated with pregnancy, several reasonable predictions can be made.88,89 First, due to their large size and hydrophilicity, monoclonal antibodies are almost exclusively confined to the blood plasma and extracellular fluid. The increase in blood volume associated with pregnancy (approximately 40%) may reduce tocilizumab concentrations, necessitating larger doses. Second, monoclonal antibodies are primarily eliminated by intracellular degradation after target binding and to a lesser degree by proteolytic catabolism. The former is related to target expression levels (e.g., IL-6R expression) which is approximately 40% higher in pregnant people compared to non-pregnant people,90 suggesting that tocilizumab may be eliminated more rapidly in pregnancy, necessitating a larger initial dose or a second administration
Maternal IgG1 does not transfer well into breastmilk, although concentrations are higher in the colostrum from mothers of preterm infants.91,92 Furthermore, IgG oral bioavailability is low due to degradation in the infant digestive tract. In keeping with these general properties of IgG, tocilizumab is excreted into breastmilk reaching peak levels 3 to 5 days after dosing, but only small amounts are detected (breastmilk-to-serum ratios less than 0.0015).93–95 No adverse effects were observed in reports of breastfed infants whose mothers were treated with tocilizumab.93–95
Venous Thromboembolism Prophylaxis
In general, pregnant women are at higher risk of venous thromboembolism (VTE).96 SARS-CoV-2 infection itself heightens the risk of VTE, especially pulmonary embolism.97 Hospitalized pregnant antenatal and postpartum patients with severe COVID-19 should receive appropriate VTE prophylaxis. Any VTE prophylaxis strategy in critically ill pregnant patients should consider the mode of delivery, whether delivery is imminent, and whether spinal or epidural anaesthesia is required.98 Therapeutic anticoagulation was evaluated across critically ill patients with COVID-19 and was not associated with a reduced need for organ support. Furthermore, it was found to increase bleeding events compared to prophylactic dose anticoagulation.99
Vaccines in Pregnancy
Given the higher severity of COVID-19 illness in the pregnant population, vaccine uptake is critical to minimize morbidity and mortality, especially with the emergence of more transmissible and virulent VOCs.100 Vaccine trials excluded pregnant and lactating individuals. However the recommendation for COVID-19 vaccination in this population is supported by observational studies, including immunogenicity,101 vaccine safety,102–104 and vaccine efficacy data.105Ontario’s Better Outcomes Registry & Network (BORN) reported preliminary findings for the period of December 14, 2020, to May 31, 2021, showing that, among 4,902 vaccinated pregnant individuals in Ontario who had already given birth, there was no increased risk for adverse pregnancy or birth outcomes when compared to unvaccinated pregnant individuals over the same period.103 Furthermore, in 130,000 pregnant individuals in the United Stated who have received the COVID-19 vaccine, no increased adverse events were reported.104 A recent retrospective cohort study of 7,530 pregnant individuals in Israel found vaccination with the Pfizer-BioNTech mRNA vaccine was associated with a significantly lower risk of SARS-CoV-2 infection after 28 days with an adjusted hazard ratio of 0.22 (95% CI 0.11 to 0.43) as compared to 7,530 matched unvaccinated pregnant individuals.105 This efficacy data is further supported by preliminary reports from the US and UK demonstrating lower rates of COVID-19 and severe COVID-19 after vaccination in pregnancy.106,107
Despite pregnant individuals in Ontario being designated a priority population for vaccination in late April 2021, vaccine uptake has been low. As of July 4, 2021, the I.C.E.S. Ontario COVID-19 dashboard shows that pregnant people have the lowest vaccine uptake as compared to all other highest-risk groups in Ontario,108 with only 53% of pregnant individuals having received at least one vaccine dose and 27% having received both vaccine doses. The lower vaccine uptake may be due hesitation to strongly recommend vaccination prior to published safety data, conflicting messaging, and uncertainty regarding safety or efficacy data among pregnant individuals. Given the heighted severity of illness and recent safety and efficacy data of the vaccine, the Society of Obstetricians and Gynecologists of Canada (SOGC) and the National Advisory Committee on Immunizations (NACI) state that pregnant individuals should be offered mRNA vaccination at any time during pregnancy or while breastfeeding, if no contraindications exist.100,109 The Centres for Disease Control (CDC) in the US has recently changed their guidance to explicitly recommend vaccination among pregnant and breastfeeding individuals.110 Informed consent should include discussion surrounding reassuring evidence on the safety and efficacy of mRNA COVID-19 vaccines in these populations as well as the risk of morbidity among pregnant individuals if unvaccinated considering VOCs.
Tailored messaging to the pregnant population is critical to increase vaccine confidence and uptake. Decision making support tools are available through the Ontario Ministry of Health (MOH) and these shared decision-making frameworks have been shown to be helpful when counselling patients.111,112
Interpretation
Based on available evidence, pregnant individuals appear to have the same incidence of infection with SARS-CoV-2 compared to non-pregnant females of the same age, but may be more likely to have asymptomatic infection. Pregnant individuals with COVID-19 are more likely to be admitted to hospital compared to age and sex matched patients with COVID-19, and also have higher rates of admission to the ICU, and greater utilization of invasive mechanical ventilation and ECMO.
This increased illness severity is potentially related to physiologic, anatomic, and immunologic changes during pregnancy. This mirrors other respiratory illnesses seen in previous pandemics. Compared to pregnant individuals without COVID-19, there was a higher incidence of maternal mortality, Cesarean delivery, and preterm birth. Fortunately, there were minimal fetal adverse effects and neonatal acquisition was reported to be low. For those with severe ARDS, transfer to a specialized centre with high-risk obstetrics, critical care obstetrical expertise, obstetrical medicine, obstetrical anaesthesia, and high-level neonatal ICUs should be considered. Telemedicine consultation can also expand access to specialist expertise if it is not locally available.
Principles of ICU supportive care for severe acute respiratory failure and ARDS management should be similar to the non-COVID-19 pregnant patient, including their candidacy for prone positioning and ECMO. In the setting of severe ARDS during late third trimester pregnancy, prior to considering prone positioning and ECMO, timing of delivery should be discussed with the obstetrical care provider, taking into consideration the physiologic impact of the fetus, gestational age of the fetus, severity of illness of the patient, and the risks of the intervention. Evidence-based medications for moderate to severe COVID-19 can be used in the pregnant patient after discussing the risks and/or benefits with the patient or substitute decision maker.
Given the higher severity of illness, predominance of VOCs, maternal morbidity and mortality associated with COVID-19 infection, and safety and efficacy of COVID-19 vaccines to date, vaccination is an important strategy to minimize morbidity and mortality in this vulnerable group. There has been low uptake of COVID-19 vaccines in the pregnant population and all proven strategies decrease hesitancy and increase uptake should be applied. A previous Science Advisory Table Brief includes strategies to minimize any barriers or hesitancy that may exist.113
There is limited information on how the emergence of SARS-CoV-2 VOCs have impacted the incidence, severity, outcomes, and management of pregnant patients with COVID-19. More data is needed to inform whether the transmission and severity is heighted in this population compared to the non-pregnant population. An upcoming CANCOVID report will evaluate the impact of VOCs in pregnancy.
Finally, pregnant individuals were excluded from many randomized trials during the COVID-19 pandemic — likely contributing to 1) hesitancy in the initiation of life saving therapies as evidence evolved and 2) uncertainty surrounding the effectiveness of vaccines. The Ontario Provincial Council for Maternal Child Health recently highlighted the inequity that exists as a result of the exclusion of pregnant people from clinical research when they wrote: “Pregnant people deserve equity in access to therapeutic options that are informed by rigorous scientific data. Systematic exclusion of sick pregnant people from clinical trials leaves them vulnerable to limitations in access to off-label or compassionate use of therapeutics, or limits evidence-based care due to lack of information specific to pregnancy. Safe inclusion in COVID-19 clinical trials is required to provide pregnant people with equal access to treatments and vaccines during the pandemic.”114
Research in future pandemics, particularly involving respiratory illnesses, should have a streamlined approach for rapid consideration of safety and efficacy of therapies in the pregnant population. This would ensure pregnant individuals are included in the evaluation of potentially life-saving therapies. Furthermore, this should be accompanied by knowledge translation initiatives to minimize hesitancy for enrollment across care providers.
Methods Used for This Science Brief
The COVID-19 Evidence Synthesis Network performed a research evidence scan for this Science Brief, published in an Evidence Synthesis Briefing Note. The COVID-19 Evidence Synthesis Network is comprised of organizations in Ontario’s evidence synthesis and knowledge translation community who collectively provide high-quality, relevant, and timely synthesized research evidence about COVID-19. The Methods for the evidence scan can be found in the methods section of the Briefing Note. The evidence scan was last updated on May 6, 2021.115 An updated literature review was conducted on July 5, 2021, by the first author to identify any additional relevant articles.
References
- Harris JW. Influenza occurring in pregnant women: A statistical study of thirteen hundred and fifty cases. J Am Med Assoc. 1919;72(14):978-980. https://doi.org/10.1001/jama.1919.02610140008002
- Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78(6):1172-1175. https://doi.org/10.1016/0002-9378(59)90570-8
- Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 Influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303(15):1517-1525. https://doi.org/10.1001/jama.2010.479
- Mascio DD, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2(2). https://doi.org/10.1016/j.ajogmf.2020.100107
- Mark EG, McAleese S, Golden WC, et al. Coronavirus disease 2019 in pregnancy and outcomes among pregnant women and neonates: A literature review. Pediatr Infect Dis J. 2021;40(5):473-478. https://doi.org/10.1097/INF.0000000000003102
- Money D. Canadian surveillance of COVID-19 in pregnancy: Epidemiology, maternal and infant outcomes.; 2021. http://med-fom-ridprogram.sites.olt.ubc.ca/files/2021/06/CANCOVID_Preg-Report-4-ON-BC-QC-MB-AB_FINAL.pdf
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ. 2020;370. https://doi.org/10.1136/bmj.m3320
- Papapanou M, Papaioannou M, Petta A, et al. Maternal and neonatal characteristics and outcomes of COVID-19 in pregnancy: An overview of systematic reviews. Int J Environ Res Public Health. 2021;18(2):596. https://doi.org/10.3390/ijerph18020596
- Novoa RH, Quintana W, Llancarí P, Urbina-Quispe K, Guevara-Ríos E, Ventura W. Maternal clinical characteristics and perinatal outcomes among pregnant women with coronavirus disease 2019. A systematic review. Travel Med Infect Dis. 2021;39:101919. https://doi.org/10.1016/j.tmaid.2020.101919
- Pettirosso E, Giles M, Cole S, Rees M. COVID-19 and pregnancy: A review of clinical characteristics, obstetric outcomes and vertical transmission. Aust N Z J Obstet Gynaecol. 2020;60(5):640-659. https://doi.org/10.1111/ajo.13204
- Joseph NT, Rasmussen SA, Jamieson DJ. The effects of COVID-19 on pregnancy and implications for reproductive medicine. Fertil Steril. 2021;115(4):824-830. https://doi.org/10.1016/j.fertnstert.2020.12.032
- Vouga M, Favre G, Martinez-Perez O, et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci Rep. 2021;11(1):13898. https://doi.org/10.1038/s41598-021-92357-y
- Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817-826. https://doi.org/10.1001/jamapediatrics.2021.1050
- Fisman DN, Tuite AR. Progressive increase in virulence of novel SARS-CoV-2 variants in Ontario, Canada, from December to July, 2021. medRxiv. Published online July 7, 2021:2021.07.05.21260050. https://doi.org/10.1101/2021.07.05.21260050
- Knight M, Ramakrishnan R, Bunch K, et al. Females in hospital with SARS-CoV-2 infection, the association with pregnancy and pregnancy outcomes: A UKOSS/ISARIC/CO-CIN investigation.; 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/977287/s1171-ukoss-isaric-co-cin-covid-19-young-females-pregnancy-report.pdf
- Kadiwar S, Smith JJ, Ledot S, et al. Were pregnant women more affected by COVID-19 in the second wave of the pandemic? The Lancet. 2021;397(10284):1539-1540. https://doi.org/10.1016/S0140-6736(21)00716-9
- D’Antonio F, Sen C, Mascio DD, et al. Maternal and perinatal outcomes in high compared to low risk pregnancies complicated by severe acute respiratory syndrome coronavirus 2 infection (phase 2): The World Association of Perinatal Medicine working group on coronavirus disease 2019. Am J Obstet Gynecol MFM. 2021;3(4):100329. https://doi.org/10.1016/j.ajogmf.2021.100329
- Turan O, Hakim A, Dashraath P, Jeslyn WJL, Wright A, Abdul-Kadir R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: A systematic review. Int J Gynecol Obstet. 2020;151(1):7-16. https://doi.org/10.1002/ijgo.13329
- Papageorghiou AT, Deruelle P, Gunier RB, et al. Preeclampsia and COVID-19: Results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. Published online June 26, 2021. https://doi.org/10.1016/j.ajog.2021.05.014
- Dong L, Pei S, Ren Q, et al. Evaluation of vertical transmission of SARS-CoV-2 in utero: Nine pregnant women and their newborns. Placenta. 2021;111:91-96. https://doi.org/10.1016/j.placenta.2021.06.007
- Kirtsman M, Diambomba Y, Poutanen SM, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ Can Med Assoc J J Assoc Medicale Can. 2020;192(24):E647-E650. https://doi.org/10.1503/cmaj.200821
- Shaiba LA, Hadid A, Altirkawi KA, et al. Case report: Neonatal multi-system inflammatory syndrome associated with SARS-CoV-2 exposure in two cases from Saudi Arabia. Front Pediatr. Published online 2021. https://doi.org/10.3389/fped.2021.652857
- Pawar R, Gavade V, Patil N, et al. Neonatal multisystem inflammatory syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: A case series. Children. 2021;8(7):572. https://doi.org/10.3390/children8070572
- Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193(16). https://doi.org/10.1503/cmaj.202604
- Tang J, Li N, Chen X, et al. Prenatal hypoxia induced dysfunction in cerebral arteries of offspring rats. J Am Heart Assoc. 2017;6(10). https://doi.org/10.1161/JAHA.117.006630
- Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159-2168. https://doi.org/10.1056/NEJMoa1214103
- Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S280-S288. https://doi.org/10.1513/AnnalsATS.201704-343OT
- Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of intensive care medicine/society of critical care medicine clinical practice guideline: Mechanical ventilation in adult patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2017;195(9):1253-1263. https://doi.org/10.1164/rccm.201703-0548ST
- Coronavirus (COVID-19) infection in pregnancy. Published online February 19, 2021. Accessed August 26, 2021. https://www.rcog.org.uk/globalassets/documents/guidelines/2021-02-19-coronavirus-covid-19-infection-in-pregnancy-v13.pdf
- Tolcher MC, McKinney JR, Eppes CS, et al. Prone positioning for pregnant women with hypoxemia due to coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2020;136(2):259-261. chttps://www.rcog.org.uk/globalassets/documents/guidelines/2021-02-19-coronavirus-covid-19-infection-in-pregnancy-v13.pdf
- Samanta S, Samanta S, Wig J, Baronia AK. How safe is the prone position in acute respiratory distress syndrome at late pregnancy? Am J Emerg Med. 2014;32(6). https://doi.org/10.1016/j.ajem.2013.12.021
- Jacobson J, Antony K, Beninati M, Alward W, Hoppe KK. Use of dexamethasone, remdesivir, convalescent plasma and prone positioning in the treatment of severe COVID-19 infection in pregnancy: A case report. Case Rep Womens Health. 2021;29. https://doi.org/10.1016/j.crwh.2020.e00273
- Vibert F, Kretz M, Thuet V, et al. Prone positioning and high-flow oxygen improved respiratory function in a 25-week pregnant woman with COVID-19. Eur J Obstet Gynecol Reprod Biol. 2020;250:257-258. https://doi.org/10.1016/j.ejogrb.2020.05.022
- Jüni P, Odutayo A, Allen U, et al. Dexamethasone in patients hospitalized for COVID-19. Sci Briefs Ont COVID-19 Sci Advis Table. 2020;1(1). https://doi.org/10.47326/ocsat.2020.01.01.1.0
- Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
- Bandoli G, Palmsten K, Forbess Smith CJ, Chambers CD. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum Dis Clin North Am. 2017;43(3):489-502. https://doi.org/10.1016/j.rdc.2017.04.013
- Czeizel AE, Rockenbauer M. Population-based case-control study of teratogenic potential of corticosteroids. Teratology. 1997;56(5):335-340. https://doi.org/10.1002/(SICI)1096-9926(199711)56:5<335::AID-TERA7>3.0.CO;2-W
- Hviid A, Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ Can Med Assoc J J Assoc Medicale Can. 2011;183(7). https://doi.org/10.1503/cmaj.101063
- Mossey PA, Little J, Munger RG, Dixon MJ, Shaw WC. Cleft lip and palate. Lancet Lond Engl. 2009;374(9703):1773-1785. https://doi.org/10.1016/S0140-6736(09)60695-4
- Skuladottir H, Wilcox AJ, Ma C, et al. Corticosteroid use and risk of orofacial clefts. Birt Defects Res A Clin Mol Teratol. 2014;100(6):499-506. https://doi.org/10.1002/bdra.23248
- Beitins IZ, Bayard F, Ances IG, Kowarski A, Migeon CJ. The transplacental passage of prednisone and prednisolone in pregnancy near term. J Pediatr. 1972;81(5):936-945. https://doi.org/10.1016/S0022-3476(72)80547-X
- Lamontagne F, Briel M, Guyatt GH, Cook DJ, Bhatnagar N, Meade M. Corticosteroid therapy for acute lung injury, acute respiratory distress syndrome, and severe pneumonia: A meta-analysis of randomized controlled trials. J Crit Care. 2010;25(3):420-435. https://doi.org/10.1016/j.jcrc.2009.08.009
- Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the diagnosis and management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med. 2017;45(12):2078-2088. https://doi.org/10.1097/CCM.0000000000002737
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: Results of a randomized controlled trial. Chest. 2007;131(4):954-963. https://doi.org/10.1378/chest.06-2100
- Meduri GU, Bridges L, Shih M-C, Marik PE, Siemieniuk RAC, Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: Analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016;42(5):829-840. https://doi.org/10.1007/s00134-015-4095-4
- Crowther CA, Ashwood P, Andersen CC, et al. Maternal intramuscular dexamethasone versus betamethasone before preterm birth (ASTEROID): A multicentre, double-blind, randomised controlled trial. Lancet Child Adolesc Health. 2019;3(11):769-780. https://doi.org/10.1016/S2352-4642(19)30292-5
- Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021;21(1):337. https://doi.org/10.1186/s12879-021-06045-3
- Saad AF, Chappell L, Saade GR, Pacheco LD. Corticosteroids in the management of pregnant patients with Coronavirus disease (COVID-19). Obstet Gynecol. 2020;136(4):823-826. https://doi.org/10.1097/AOG.0000000000004103
- Morris AM, Juni P, Odutayo A, et al. Remdesivir for hospitalized patients with COVID-19. Sci Briefs Ont COVID-19 Sci Advis Table. 2021;2(27). https://doi.org/10.47326/ocsat.2021.02.27.1.0
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. Published online May 22, 2020. https://doi.org/10.1056/NEJMoa2007764
- Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383(19):1827-1837. https://doi.org/10.1056/NEJMoa2015301
- Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. Published online August 21, 2020. https://doi.org/10.1001/jama.2020.16349
- Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. Published online 2020. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31022-9/fulltext
- Pan H, Peto R, Karim QA, et al. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. Published online October 15, 2020:2020.10.15.20209817. https://doi.org/10.1101/2020.10.15.20209817
- Dubinion J, Ghantous H, Birnkrant DB, Kim C. Pharmacology/toxicology NDA review and evaluation. VekluryTM(Remdesivir [RDV]/ GS-5734), non-clinical reviews. Center for Drug Evaluation and Research; 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/214787Orig1s000PharmR.pdf
- Chan-Tack K. Pharmacology/toxicology NDA review and evaluation. VekluryTM(Remdesivir [RDV]/ GS-5734), clinical reviews. Center for Drug Evaluation and Research; 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/214787Orig1s000MedR.pdf
- Burwick RM, Yawetz S, Stephenson KE, et al. Compassionate use of remdesivir in pregnant women with severe Coronavirus disease 2019. Clin Infect Dis. Published online October 8, 2020. https://doi.org/10.1093/cid/ciaa1466
- Anderson J, Schauer J, Bryant S, Graves CR. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: A case report. Case Rep Womens Health. 2020;27. https://doi.org/10.1016/j.crwh.2020.e00221
- Dande R, Qureshi A, Persaud K, Puri C, Zulfiqar S, Awasthi S. Remdesivir in a pregnant patient with COVID-19 pneumonia. J Community Hosp Intern Med Perspect. 2021;11(1):103-106. https://doi.org/10.1080/20009666.2020.1857510
- Maldarelli GA, Savage M, Mazur S, Oxford-Horrey C, Salvatore M, Marks KM. Remdesivir treatment for severe COVID-19 in third-trimester pregnancy: Case report and management discussion. Open Forum Infect Dis. 2020;7(9). https://doi.org/10.1093/ofid/ofaa345
- McCoy JA, Short WR, Srinivas SK, Levine LD, Hirshberg A. Compassionate use of remdesivir for treatment of severe coronavirus disease 2019 in pregnant women at a United States academic center. Am J Obstet Gynecol MFM. 2020;2(3). https://doi.org/10.1016/j.ajogmf.2020.100164
- Naqvi M, Zakowski P, Glucksman L, Smithson S, Burwick RM. Tocilizumab and remdesivir in a pregnant patient with Coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2020;136(5):1025-1029. https://doi.org/10.1097/AOG.0000000000004050
- Nasrallah S, Nguyen AQ, Hitchings L, et al. Pharmacological treatment in pregnant women with moderate symptoms of coronavirus disease 2019 (COVID-19) pneumonia. J Matern Fetal Neonatal Med. Published online March 26, 2021. https://doi.org/10.1080/14767058.2021.1903426
- Pelayo J, Pugliese G, Salacup G, et al. Severe COVID-19 in third trimester pregnancy: Multidisciplinary approach. Case Rep Crit Care. 2020;2020. https://doi.org/10.1155/2020/8889487
- Igbinosa I, Miller S, Bianco K, et al. Use of remdesivir for pregnant patients with severe novel coronavirus disease 2019. Am J Obstet Gynecol. 2020;223(5):768-770. https://doi.org/10.1016/j.ajog.2020.08.001
- Jorgensen SCJ, Kebriaei R, Dresser LD. Remdesivir: Review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacother J Hum Pharmacol Drug Ther. 2020;40(7):659-671. https://doi.org/10.1002/phar.2429
- International Maternal Pediatric Adolescent AIDS Clinical Trials Network. Pharmacokinetics and safety of remdesivir for treatment of COVID-19 in pregnant and non-pregnant women in the United States. Protocol Version 2.0. IMPAACT 2032; 2020. https://www.impaactnetwork.org/sites/default/files/2021-04/IMPAACT_2032_PROTOCOL_FINAL_V2.0_18DEC2020_CM_1.pdf
- Drugs and Lactation Database (LactMed). Remdesivir. Drugs and Lactation Database (LactMed). Published 2006.http://www.ncbi.nlm.nih.gov/books/NBK556881/
- Frauenfelder C, Brierley J, Whittaker E, Perucca G, Bamford A. Infant with SARS-CoV-2 infection causing severe lung disease treated with remdesivir. Pediatrics. 2020;146(3). https://doi.org/10.1542/peds.2020-1701
- Dörnemann J, Burzio C, Ronsse A, et al. First newborn baby to receive experimental therapies survives Ebola virus disease. J Infect Dis. 2017;215(2):171-174. https://doi.org/10.1093/infdis/jiw493
- Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group. Clinical practice guideline summary: Recommended drugs and biologics in adult patients with COVID-19. Ont COVID-19 Sci Advis Table. 2021;Version 1.0. https://doi.org/10.47326/ocsat.cpg.2021.1.0
- Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): Preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. Published online February 11, 2021:2021.02.11.21249258. https://doi.org/10.1101/2021.02.11.21249258
- Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: A randomized clinical trial. JAMA Intern Med. 2020;181(1):32. https://doi.org/10.1001/jamainternmed.2020.6820
- Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: A randomized clinical trial. JAMA Intern Med. 2021;181(1):24. https://doi.org/10.1001/jamainternmed.2020.6615
- Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20-30. https://doi.org/10.1056/NEJMoa2030340
- Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of Tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. https://doi.org/10.1056/NEJMoa2028836
- Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84.https://doi.org/10.1136/bmj.n84
- Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19 – preliminary report. medRxiv. Published online January 9, 2021:2021.01.07.21249390. https://doi.org/10.1101/2021.01.07.21249390
- Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. https://doi.org/10.1056/NEJMoa2028700
- Soin AS, Kumar K, Choudhary NS, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): An open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021;9(5):511-521. https://doi.org/10.1016/S2213-2600(21)00081-3
- Stewart J. Developmental toxicity testing of monoclonal antibodies: An enhanced pre- and postnatal study design option. Reprod Toxicol Elmsford N. 2009;28(2):220-225. https://doi.org/10.1016/j.reprotox.2009.04.002
- Brown PC. ActemraTM(Tocilizumab). Pharmacology NDA review and evaluation. Centre for Drug Evaluation and Research; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/125276s000PharmR.pdf
- Hoeltzenbein M, Beck E, Rajwanshi R, et al. Tocilizumab use in pregnancy: Analysis of a global safety database including data from clinical trials and post-marketing data. Semin Arthritis Rheum. 2016;46(2):238-245. https://doi.org/10.1016/j.semarthrit.2016.05.004
- Skorpen CG, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795-810. https://doi.org/10.1136/annrheumdis-2015-208840
- Hyoun SC, Običan SG, Scialli AR. Teratogen update: Methotrexate. Birt Defects Res A Clin Mol Teratol. 2012;94(4):187-207. https://doi.org/10.1002/bdra.23003
- Jiménez-Lozano I, Caro-Teller JM, Fernández-Hidalgo N, et al. Safety of tocilizumab in COVID-19 pregnant women and their newborn: A retrospective study. J Clin Pharm Ther. 2021;46(4):1062-1070. https://doi.org/10.1111/jcpt.13394
- San-Juan R, Barbero P, Fernández-Ruiz M, et al. Incidence and clinical profiles of COVID-19 pneumonia in pregnant women: A single-centre cohort study from Spain. EClinicalMedicine. 2020;23. https://doi.org/10.1016/j.eclinm.2020.100407
- Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacomet Syst Pharmacol. 2017;6(9):576-588. https://doi.org/10.1002/psp4.12224
- Costantine M. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. https://doi.org/10.3389/fphar.2014.00065
- Matsuzaki N, Neki R, Sawai K, et al. Soluble interleukin-6 (IL-6) receptor in the sera of pregnant women forms a complex with IL-6 and augments human chorionic gonadotropin production by normal human trophoblasts through binding to the IL-6 signal transducer. J Clin Endocrinol Metab. 1995;80(10):2912-2917. https://doi.org/10.1210/jcem.80.10.7559874
- Koenig A, de Albuquerque Diniz EM, Barbosa SFC, Vaz FAC. Immunologic factors in human milk: the effects of gestational age and pasteurization. J Hum Lact Off J Int Lact Consult Assoc. 2005;21(4):439-443. https://doi.org/10.1177/0890334405280652
- Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3(4):442-474. https://doi.org/10.3390/nu3040442
- Saito J, Yakuwa N, Kaneko K, et al. Clinical application of the dried milk spot method for measuring tocilizumab concentrations in the breast milk of patients with rheumatoid arthritis. Int J Rheum Dis. 2019;22(6):1130-1137. https://doi.org/10.1111/1756-185X.13557
- Saito J, Yakuwa N, Kaneko K, et al. Tocilizumab during pregnancy and lactation: drug levels in maternal serum, cord blood, breast milk and infant serum. Rheumatology. 2019;58(8):1505-1507. https://doi.org/10.1093/rheumatology/kez100
- Saito J, Yakuwa N, Takai C, et al. Tocilizumab concentrations in maternal serum and breast milk during breastfeeding and a safety assessment in infants: A case study. Rheumatology. 2018;57(8):1499-1501. https://doi.org/10.1093/rheumatology/key091
- Middleton P, Shepherd E, Gomersall JC. Venous thromboembolism prophylaxis for women at risk during pregnancy and the early postnatal period. Cochrane Database Syst Rev. 2021;(3). https://doi.org/10.1002/14651858.CD001689.pub4
- Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152-160. https://doi.org/10.1016/j.thromres.2020.05.039
- Leffert LR, Dubois HM, Butwick AJ, Carvalho B, Houle TT, Landau R. Neuraxial anesthesia in obstetric patients receiving thromboprophylaxis with unfractionated or low-molecular-weight heparin: A systematic review of spinal epidural hematoma. Anesth Analg. 2017;125(1):223-231. https://doi.org/10.1213/ANE.0000000000002173
- The REMAP-CAP, ACTIV-4a, ATTACC. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. Published online August 4, 2021. https://doi.org/10.1056/NEJMoa2103417
- Public Health Agency of Canada. National Advisory Committee on Immunization (NACI): statements and publications. Canada.ca. Published October 12, 2017. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci.html
- Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am J Obstet Gynecol. Published online March 25, 2021. https://doi.org/10.1016/j.ajog.2021.03.023
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. Published online April 21, 2021. https://doi.org/10.1056/NEJMoa2104983
- Better Outcomes Registry & Network (BORN) Ontario. COVID-19 Vaccination during Pregnancy in Ontario.; 2021. https://www.bornontario.ca/en/whats-happening/resources/Documents/COVID-19-Vaccination-During-Pregnancy-in-Ontario-Report-1—FINAL.pdf
- Centers for Disease Control and Prevention. V-safe COVID-19 vaccine pregnancy registry. CDC. Published February 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html
- Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. Published online July 12, 2021. https://doi.org/10.1001/jama.2021.11035
- Theiler RN, Wick M, Mehta R, Weaver A, Virk A, Swift M. Pregnancy and Birth Outcomes after SARS-CoV-2 Vaccination in Pregnancy. Obstetrics and Gynecology; 2021. https://doi.org/10.1101/2021.05.17.21257337
- Royal College of Obstetricians and Gynaecologists (RCOG). COVID unlocking will create ‘perfect storm’ for pregnant women, say maternity colleges. Royal College of Obstetricians & Gynaecologists. Published July 15, 2021. https://www.rcog.org.uk/en/news/covid-unlocking-will-create-perfect-storm-for-pregnant-women-say-maternity-colleges/
- IC/ES. COVID-19 Dashboard. Published April 6, 2021. Accessed April 7, 2021. https://www.ices.on.ca/DAS/AHRQ/COVID-19-Dashboard
- Poliquin V, Castillo E, Boucoiran I, et al. SOGCS statement on COVID-19 vaccination in pregnancy.; 2021. https://sogc.org/en/content/featured-news/SOGC_Statement_on_COVID-19_Vaccination_in_Pregnancy.aspx
- COVID-19 vaccines while pregnant or breastfeeding. Centers for Disease Control and Prevention. Published August 11, 2021. Accessed August 26, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
- Ministry of Health. COVID-19 Vaccination: Special populations – vaccination in pregnancy & breastfeeding patient decision-making tool.; 2021:7. https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/COVID-19_vaccination_pregnancy_decision_making_support_tool.pdf
- Zipursky JS, Greenberg RA, Maxwell C, Bogler T. Pregnancy, breastfeeding and the SARS-CoV-2 vaccine: An ethics-based framework for shared decision-making. CMAJ. 2021;193(9):E312-E314. https://doi.org/10.1503/cmaj.202833
- Presseau J, Arnason T, Buchan JL, et al. Strategies to support Ontarians’ capability, opportunity, and motivation for COVID-19 vaccination. Sci Briefs Ont COVID-19 Sci Advis Table. 2021;2(36). https://doi.org/10.47326/ocsat.2021.02.36.1.0
- Provincial Council for Maternal and Child Health. Recommendations to address gaps in prenatal care system. Report from the COVID-19 Prenatal Care Task Force.; 2021. https://www.pcmch.on.ca/wp-content/uploads/2021/01/FINAL-2021_01_13-PCMCH-Recommendations-for-Prenatal-System-Gaps.pdf
- Research, Analysis, and Evaluation Branch. Evidence synthesis briefing note: Incidence, severity and management of COVID-19 across acutely ill COVID-19 obstetrical patients. COVID-19 Evidence Synthesis Network; 2021. https://esnetwork.ca/wp-content/uploads/2021/05/ESBN-on-the-Impact-of-COVID-19-on-Obstetrical-Patients_19-MAY-2021.pdf
Document Information & Citation
Author Contributions: LM, SL, WW conceived the Science Brief. LM wrote the first draft of the Science Brief. All authors revised the Science Brief critically for important intellectual content and approved the final version.
Citation: Munshi L, Wright JK, Zipursky J, et al. The incidence, severity, and management of COVID-19 in critically ill pregnant individuals. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2021;2(43). https://doi.org/10.47326/ocsat.2021.02.43.1.0
Author Affiliations: The affiliations of the members of the Ontario COVID-19 Science Advisory Table can be found at https://covid19-sciencetable.ca/.
Declarations of Interest: The declarations of interest of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners can be found at https://covid19-sciencetable.ca/. The declarations of interest of external authors can be found under Additional Resources.
Copyright: 2021 Ontario COVID-19 Science Advisory Table. This is an open access document distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original work is properly cited.
The views and findings expressed in this Science Brief are those of the authors and do not necessarily reflect the views of all of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners.
